CARDIAC DISEASE IS THE LEADING CAUSE OF MATERNAL DEATH IN THE U.S.
CONTENTS
- General Cardio-obstetric Resources
- Maternal Cardiac Arrest
- Review of Normal CV Changes in Pregnancy
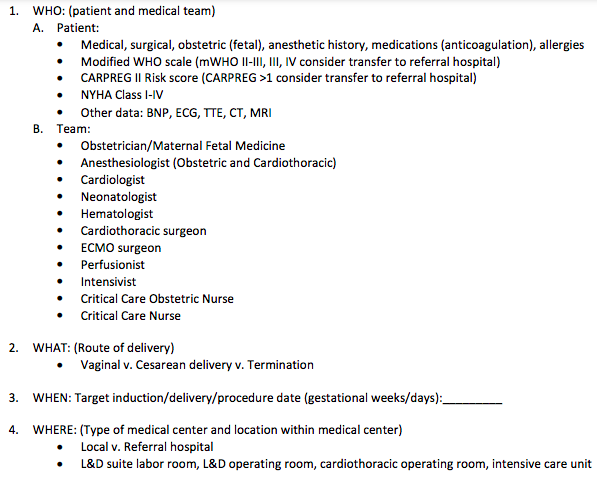
- mWHO classification of cardiac risk in pregnancy, and considerations for high-risk cardiopulmonary patient delivery planning
- Arryhthmia Management
- Ischemic Heart Disease/SCAD/MI in Pregnancy
- Cardiomyopathy in Pregnancy: DCM, PPCM, HOCM
- Valvular Lesions in Pregnancy: AR, MR, AS, MS, PS, TS
- Diastolic Dysfunction in Pregnancy (Pulmonary Edema on seperate page)
- Cardio-obstetric anesthesia: CPB during pregnancy & Fetal monitoring
- Systolic Dysfunction extra notes/slides
PULMONARY HTN HAS ITS OWN PAGE: https://tuohytime.com/home/pulmonary-htn/
General Cardio-obstetric Resources
European Society for Cardiology (ESC) 2018 Pregnancy Guidelines The holy grail of concise cardio-obstetric guidelines
ESC guidelines PDF:
https://orbi.uliege.be/bitstream/2268/290865/1/ehy340.pdf:
Great source on OB cardiac updates, along with ACOG bulletins:
California Maternal Quality Care Collaborative |
TOOLKITS for managing cardiac dysfunction/ obstetric emergencies :
Toolkits | California Maternal Quality Care Collaborative
ACOG practice bulletin *212* cardiac disease and pregnancy
AHA update on Cardiac Disease in Pregnancy
Comprehensive Review of CV Disease in Pregnancy:
Cardiac Disease in the Obstetric Patient
Obstetric Anesthesia and Heart Disease: Practical Clinical Considerations
Maternal Cardiovascular Morbidity Events Following… : Anesthesia & Analgesia
Peripartum considerations for women with cardiac disease : Current Opinion in Anesthesiology
Critical Care Obstetrics Podcast:
This MFM is great – engaging & easy to listen to – some cardiac episodes, and complex topics simple. 10/10.
The Critical Care Obstetrics Podcast
Cardionerds:
It’s a cardiology podcast but they have a cardio-obstetric series with 10 or so OB episodes – filled with content, and awesome expert speakers on every episode.
Cardionerds: A Cardiology Podcast
https://www.cardionerds.com/cardio-obstetrics/
MATERNAL CARDIAC ARREST
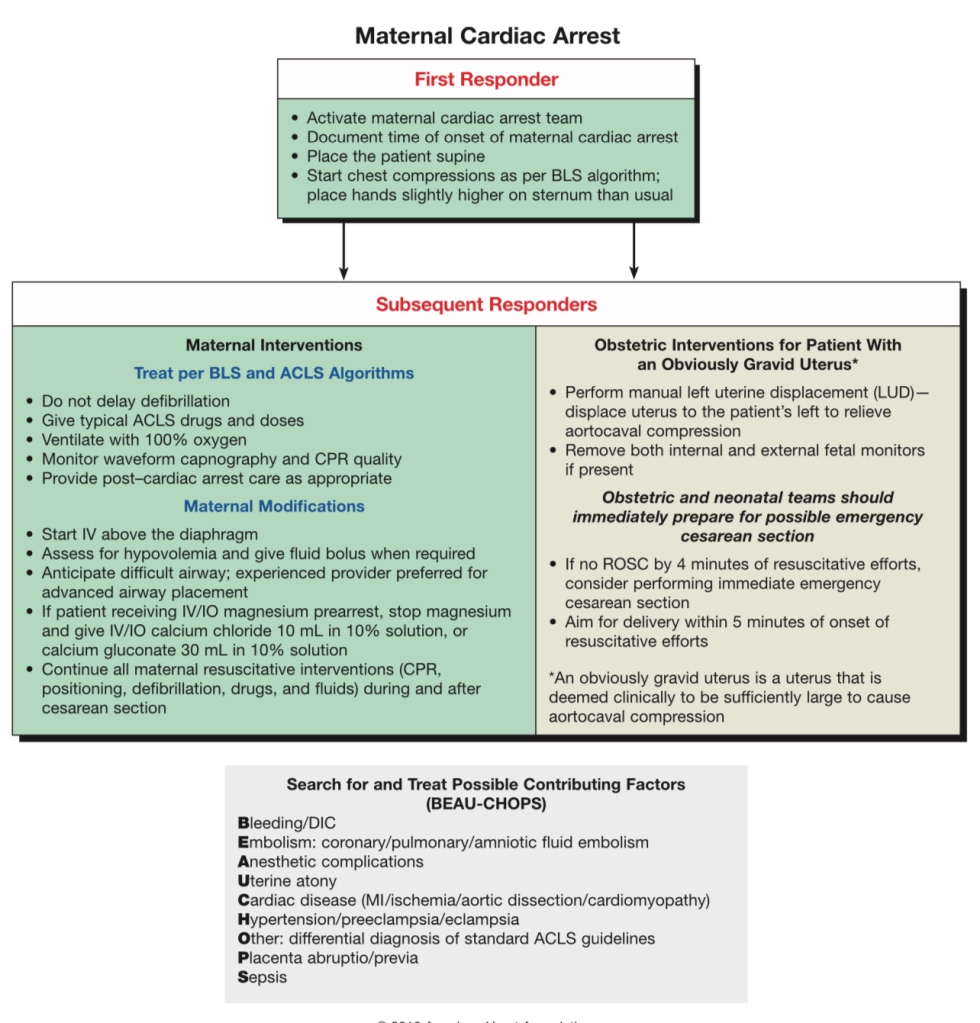
If no ROSC in 4 minutes – Emergent crash C/S no matter where you are – could be in L&D room, hallway, OR, PACU, CT scanner, triage bay, in a bed or on the floor -the literal floor. PERIMORTEM C/S OCCURS AT SITE OF ARREST. DO NOT MOVE PATIENT.
Leave patient supine & flat during CPR. A firm backboard should be used to improve compression quality. Use manual left uterine displacement. DO NOT tilt the backboard. DO NOT use blanket/roll under left hip. (The 30 degree tilt decreases force & effectiveness of chest compressions. [AHA, 2015]) . ALL IVs UPPER extremities ONLY. HUMERUS IntraOsseous line if necessary.
BEGIN CRASH C/S AT 4 MINUTES, FETUS DELIVERED BY 5 MINUTES
C/S is to facilitate Maternal CPR & resuscitation for maternal survival, not for fetal benefit, although < 5mins from arrest to delivery has improved fetal outcomes.
- Transverse or vertical incision (whatever OBGYN feels they are faster at), placenta can stay in situ until ROSC, abdomen can be packed and stapled until ROSC.
- Emergent c/s only considered if fetus > ~22-24 wks, as prior to this, fetal gestational size is not large enough to compromise maternal circulation in CPR (primary concern), and viability is unlikely, so best outcome is just restored maternal perfusion to restore fetal perfusion.
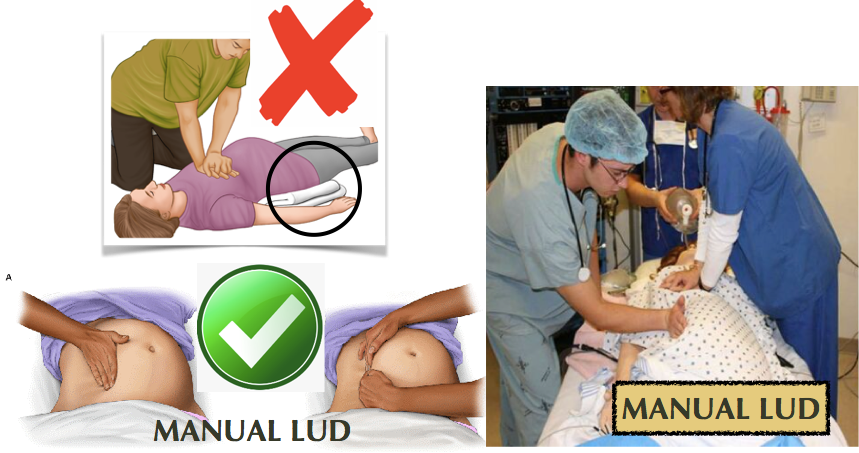
SOAP Maternal Cardiac Arrest algorithm:
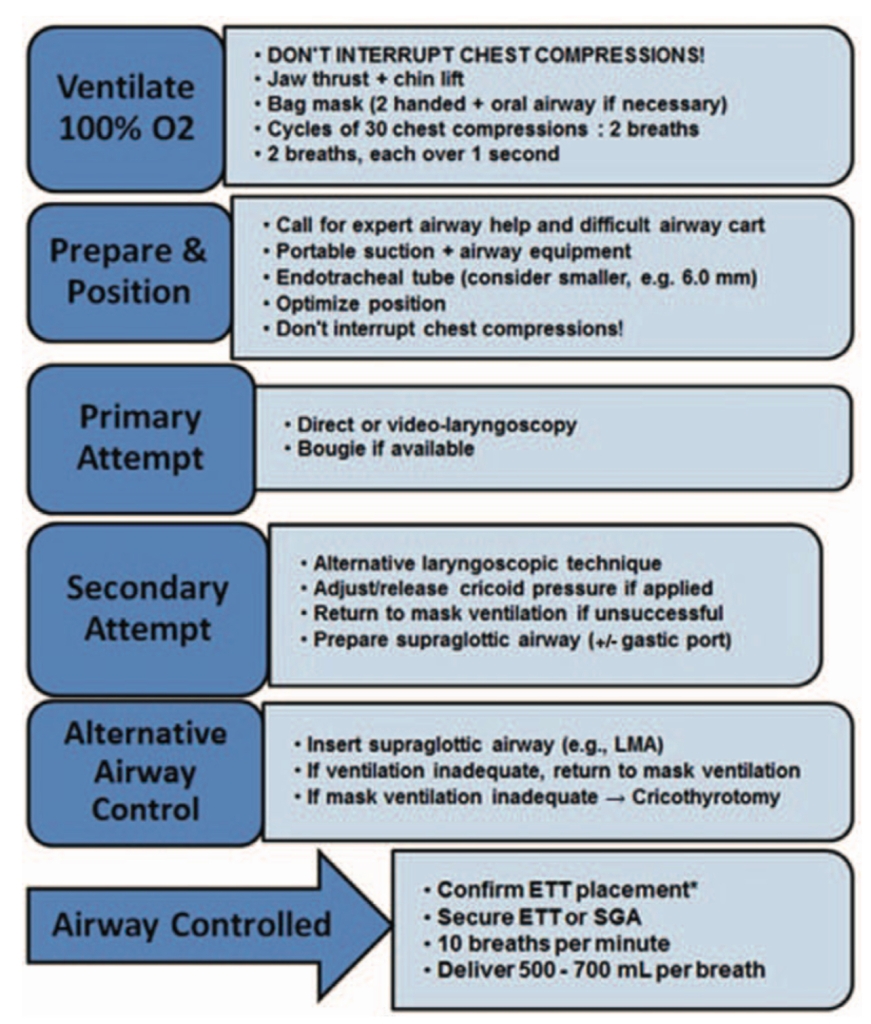
SOAP algorithm for Airway Management during CPR:
61. Case Report: Cardiac Arrest due to Peripartum Cardiomyopathy – Medical College of Wisconsin
The big players in maternal morbidity …cardiac is huge risk:
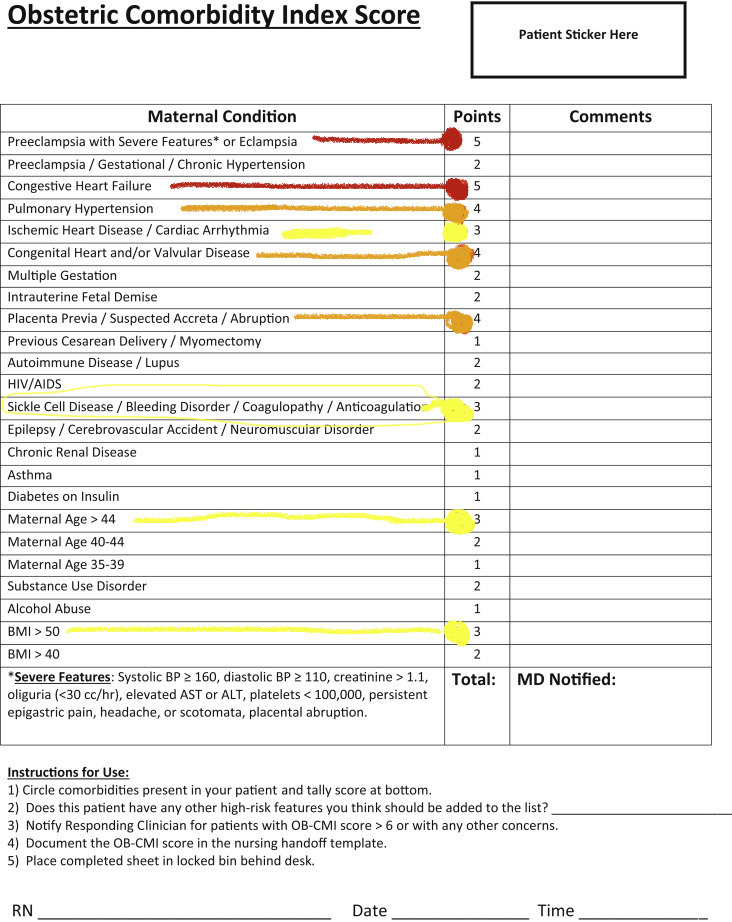
- Index score for comorbidities
- Bar graph showing totals and morbidity%
How these comorbidities contribute to requiring ICU-level care:
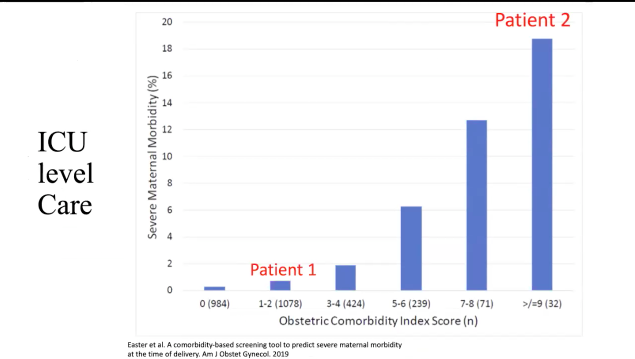

Review of NORMAL CV Changes in Pregnancy
…and imagine how a dysfunctional heart will tolerate this…
spoiler alert: it won’t!
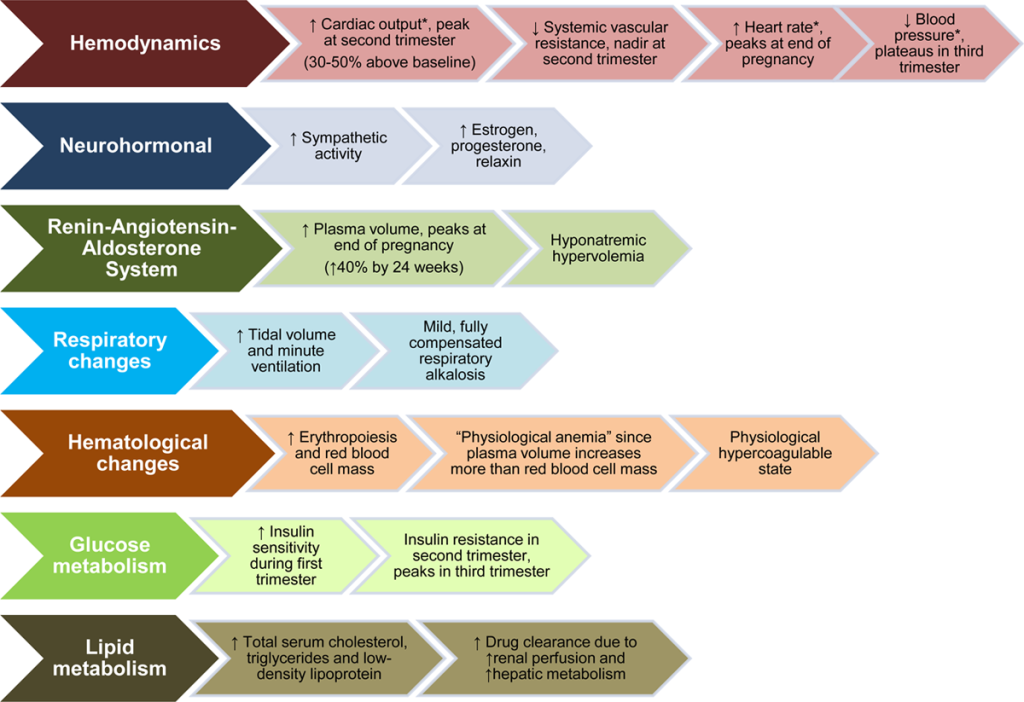
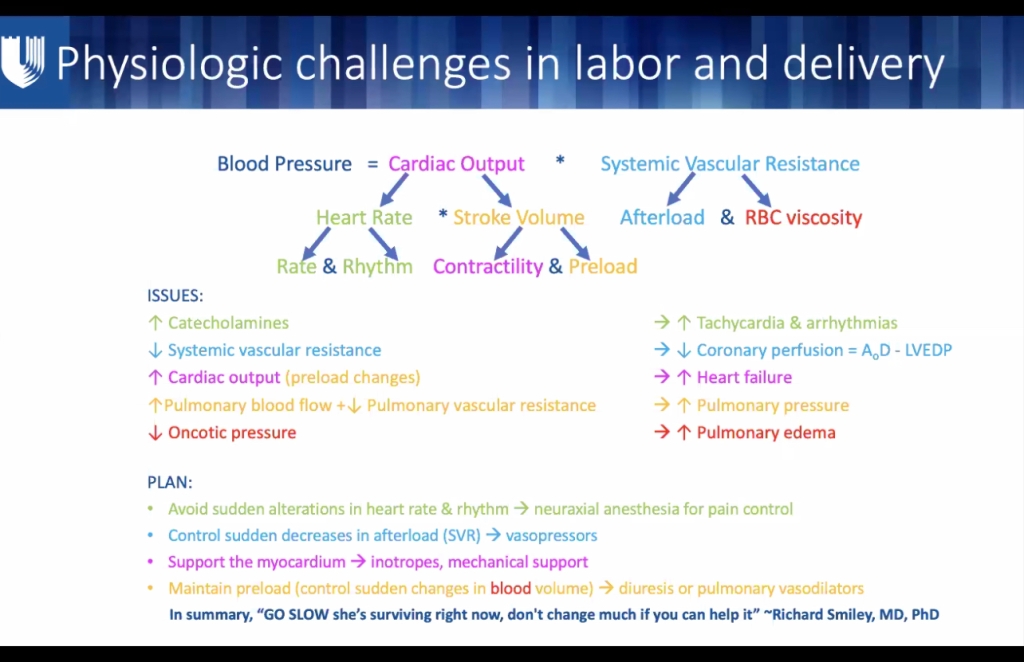
CARDIAC
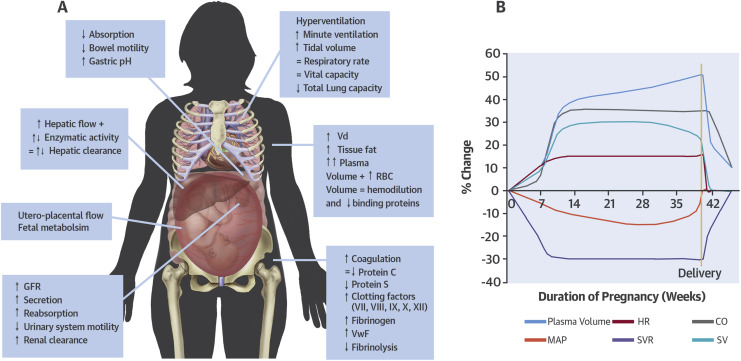
- Heart displaced cephalad & laterally 2/2 gravid uterus.
- EKG changes of pregnancy include:
- 1) sinus tachycardia
- 2) other dysrhythmias: atrial & ventricular ectopic beats
- 3) ST depression: inferior & lateral leads
- 4) T wave flattening/inversion in inferior & lateral leads
- 5) LVH
- 6) LAD 15-20o
- 7) Small Q wave, and inverted T wave in Lead III
- EKG changes of pregnancy include:
- Cardiac O2 demand higher: pregnant ARDS pt may see additional benefit of ECMO over non-pregnant pt.
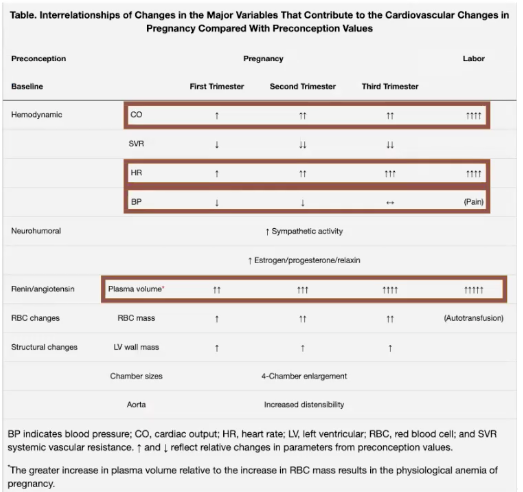
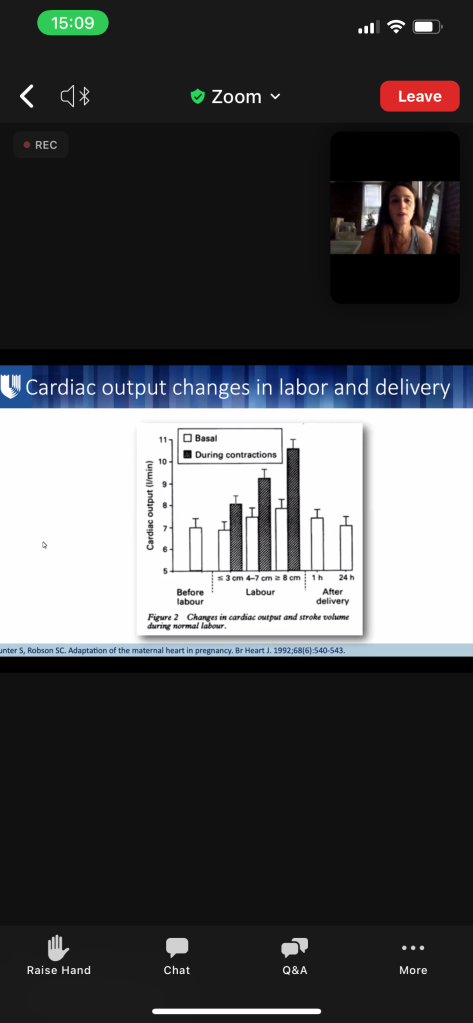
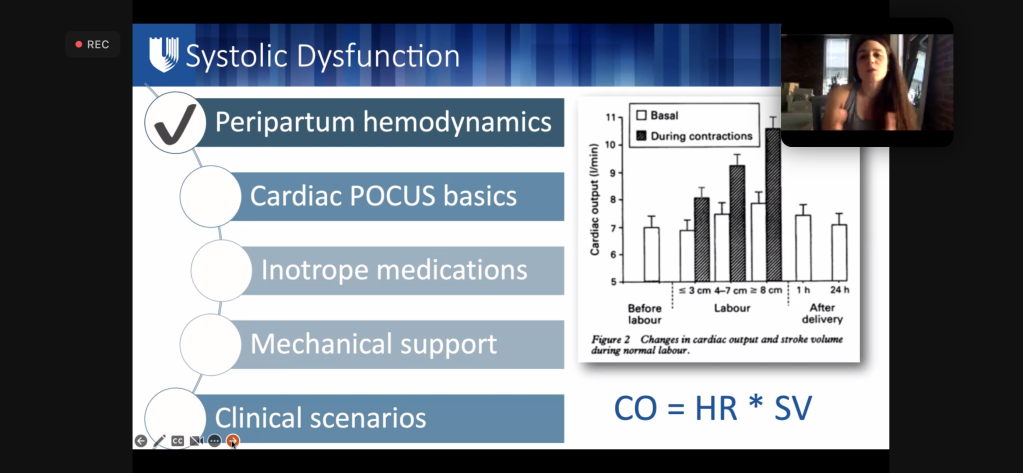
- Both SV and HR increase CO 40-50% by the 3rd TM (max at 24 weeks)
- BP drops approx 10 mmHg by 2nd TM despite 30-50% increase in intravascular volume
- Decreased SVR reduces SBP (8- 15%) and DBP (20%)
- 2/2 changes in estradiol, progesterone, nitric oxide, & prostacyclin.
- Increased venous capacitance & myocardial remodeling attenuate increased blood volume so CVP remains constant
- Relaxin: peptide hormone produced by corpus luteum, decidua, & placenta – stimulates endothelin which mediates vasodilation through N.O. synthesis
- Resistance to Angiotensin II
- Decrease in PVR to accommodate increased CO
- Plasma volume increases ~40% by 28wks, ~50% by 35wks
- But SVR & PVR decrease leaves central filling pressures unchanged.
- Decreased serum colloid-osmotic pressure placing pregnant pts at higher risk of pulmonary edema: esp in setting of increased preload (IVF/autotransfusion) and/or increased membrane permeability (PreE 2/2 endothelial damage, Magnesium) & Terbutaline (^^hydrostatic pressure from B-agonism.)
- Enlargement of both atria and both ventricles by the end of pregnancy.
- TTE & and MRI show cardiac remodeling. Atrial and ventricular chamber dilation present – usually more prominent in atria.
- LV mass increases and mild physiologic regurge can be seen in the mitral, tricuspid, and pulmonic valves.
- LV mass increases by up to 50% by 3rd TM
- Eccentric hypertrophy also noted w/ increases in septal thickness.
- Some cardiac remodeling occurs, as many of the pregnancy changes are often seen to be reversed 6 – 8 months postpartum.
- Systolic regurgitation murmurs (usually mitral or tricuspid in origin) can develop in normal pregnancy from fluid overload.
- AFTER delivery through 48-72h postpartum, CO is the highest – CO is 80% higher than before delivery.
- They’re now 150% above pre-pregnancy levels) ( ~ twice as high as term pregnancy which was already 50% above baseline)
- HIGHEST RISK OF HEART FAILURE DURING 1st WEEK POST-PARTUM
- Increased CO can be detrimental to women with baseline cardiac dysfunction: pulmonary HTN, valvular lesions (ex. stenotic lesions) or cardiomyopathies.
- Aortocaval compression decreases venous return & causes maternal HOTN —> fetal acidosis from hypo-perfusion.
- Normal hemoglobin is 12 g/dL.
- Plasma vol ^ 45% at term, RBC vol ^ 20%,
- relative dilutional anemia – so no change in MCV/MCHC
- slightly elevated WBC/leukocytosis can also be a normal finding in term pregnancy, and in labor.
- Epidural vein engorgement (Batson’s plexus) reduces the available epidural & CSF space, Causing higher neuraxial LA spread.
- POST-PARTUM:
- CO remains elevated for ~72-96h after delivery to compensate for fluid mobilization.
- Blood volume decreases ~10% in 1st week.
- SVR and HR return to normal within 2 wks postpartum
- SV and CO gradually decrease over several months.
- These persistent postpartum physiologic changes make heart failure and arrythmia continued risks in the days and weeks after delivery.
Hemodynamic and Volume Changes in Pregnancy

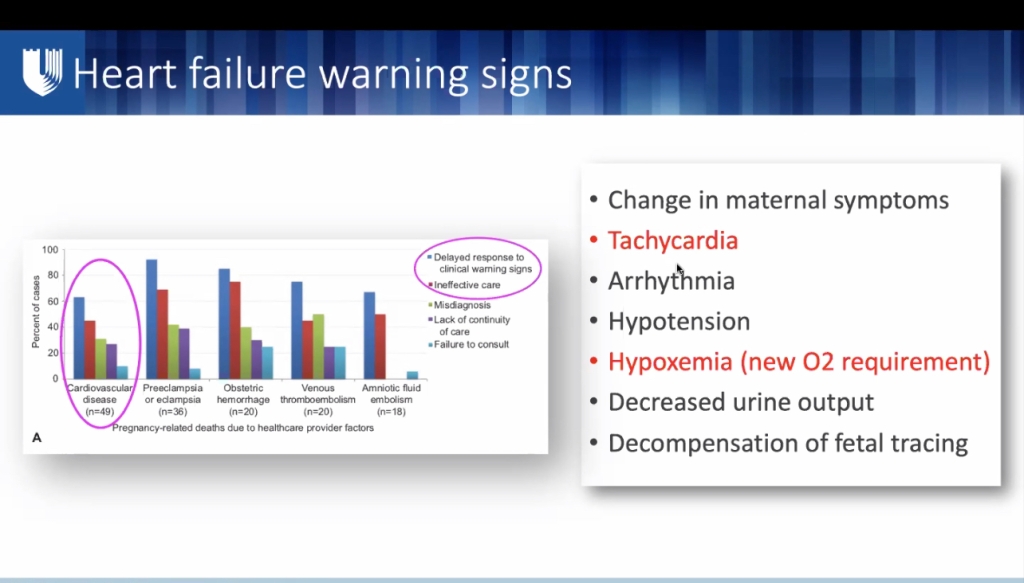
Signs & Symptoms Indicating Prompt Evaluation by Cardio-Obstetrics Team
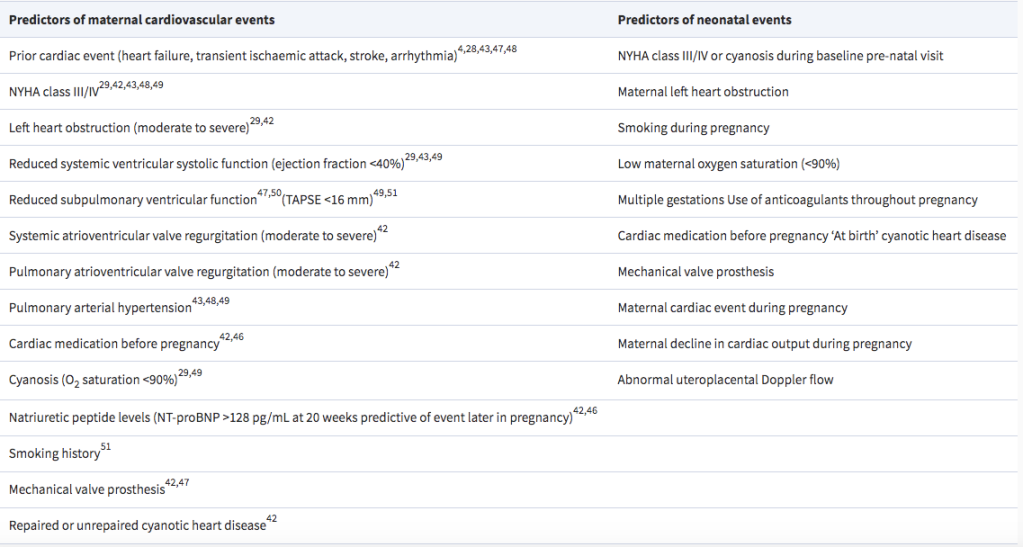
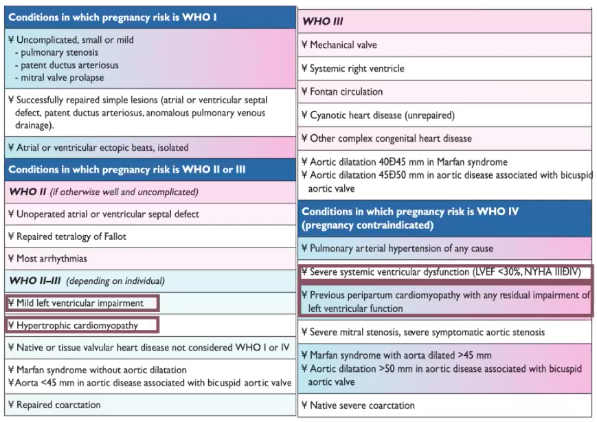
MODIFIED W.H.O (mWHO) CLASSIFICATION OF MATERNAL CV RISK
- Most common adverse cardiac events during pregnancy, delivery, & postpartum are heart failure and arrythmias.
- Common obstetric complications: premature delivery & preeclampsia.
- mWHO classification found to best classify risk over other systems (ie: CARPREG II (Cardiac Disease in Pregnancy Study), & ZAHARA)
- mWHO Risk Class IV (Pregnancy contraindicated):
- Extremely high risk of maternal mortality or severe morbidity
- If pregnancy occurs termination should be discussed.
- If pregnancy continues, care as for class III.
- Pulmonary Arterial HTN of any cause
- Severe systemic ventricular dysfunction (LVEF <30%, NYHA III-IV)*
- Previous PPCM with any residual impairment of LV function
- Severe symptomatic mitral or aortic stenosis
- Marfan syndrome with aorta dilated >45 mm
- Aortic dilation >50 mm in aortic disease assc w/ bicuspid aortic valve
- Native severe Coarctation
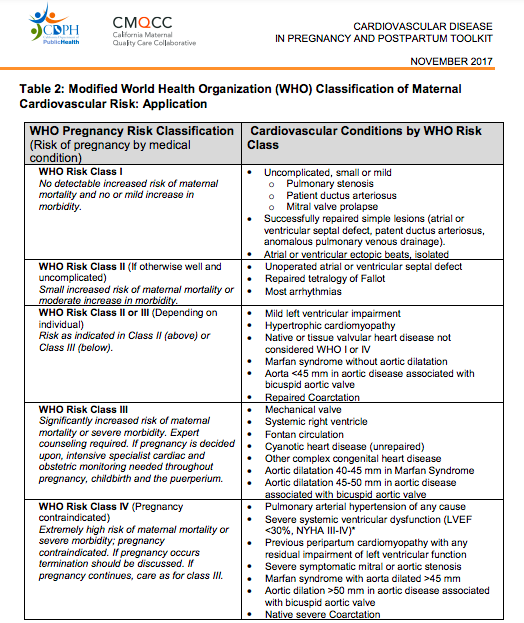
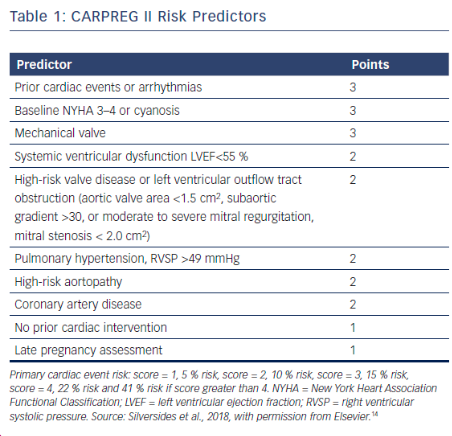
Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study
Table 1. Risks & HD Goals for mWHO Class III and IV Lesions



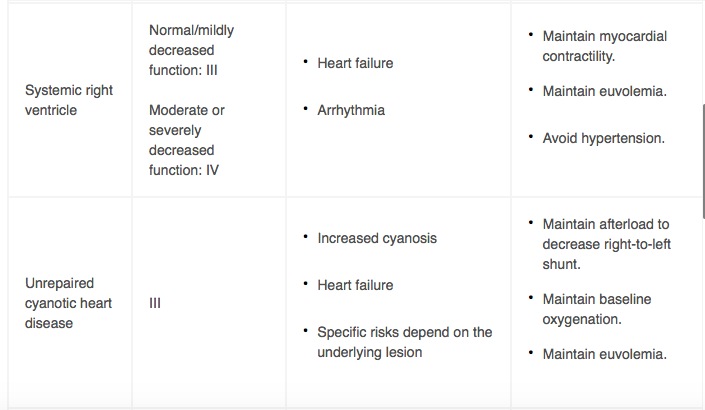

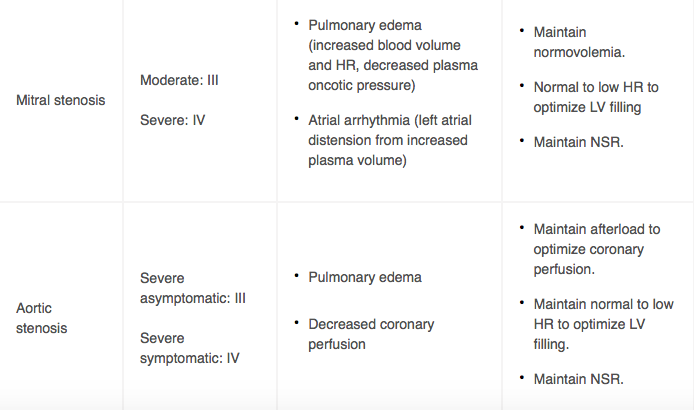






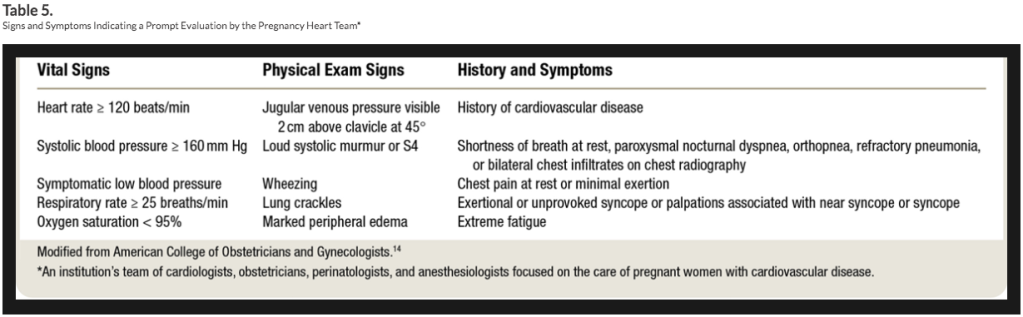
HIGH-RISK DELIVERY PLANNING CONSIDERATIONS
Pts who require a low HR or at high risk of arrhythmia should not receive epi in the test dose, as inadvertent IV epi could be deleterious. Fentanyl, 50 µg, is an adequate epidural test dose.
- Vaginal delivery with good neuraxial anesthesia is well-accepted as the preferred mode of delivery for women with cardiac disease.
- lesser blood loss & fluid shifts, less post-op infections & pulmonary complications, reduced metabolic demands & stress response.
- For epidurals: can skip initial 500cc fluid bolus, depending on type of cardiac disease state, and augment BP with pushes of pressors if necessary
- Valsalva maneuver in labor, however, can cause significant physiologic changes with each effort. During initial stages of Valsalva, intrathoracic pressure increases and venous return decreases, resulting in decreased preload & MAP, with compensatory increase in HR. As valsalva released, venous return restored and MAP increases, potentially exceeding baseline values.
- This drastic change in cardiac filling and output = increased shear stress to the aorta, which can be deleterious to women with preload-sensitive lesions or aortic pathology:
- Marfans w/ aortic root involvement
- Stanford Type A (proximal aortic root involvement)
- Ehler’s danlos
- During delivery, valsalva cycle of pushing lasts minutes or hours. On delivery, immediate and complete uterine contraction necessary to prevent hemorrhage.
- Uterine involution (autotransfusion~750cc) and removal of vena caval compression causes sudden increase in preload, requiring continued myocardial contraction augmentation for the hours following delivery.
- C/S is reserved for obstetric or fetal indications, or women with high-risk lesions to avoid hemodynamic stress of labor and delivery/valsalva:
- severe aortopathy or aortic dissection: Marfans with aortic root involvement, Stanford Type A (proximal aortic root involvement)
- (although afterload and HD stress reduction assc w/ epidural analgesia might be favorable for Marfans with aortic insufficiency and aortic dissection)
- recent MI
- severe heart failure during a previous delivery (PPCM or other causes)
- severe left-side (mitral/aortic) valvular stenosis
- any maternal decompensation requiring urgent delivery, or intubation for cardiopulmonary indication
- severe aortopathy or aortic dissection: Marfans with aortic root involvement, Stanford Type A (proximal aortic root involvement)
- As a T4-6 sensory level req. for C/S, sympathectomy greater than epidural labor analgesia. Spinal produces rapid, reliable block for surgery, but also significant HOTN and compensatory tachycardia 2/2 rapid decreases in preload & afterload.
- Bradycardia if LA affects cardioaccelerator fibers (T1-T4).
- Epidural anesthesia (including dural-puncture epidural, or “cardiac”CSE with intrathecal opioid w/ or w/o low-dose LA – 5mg bupi) is more HD stable while maintaining surgical anesthesia.
- Neo or levo gtt & cautious IVF admin to prevent neuraxial HOTN during C/S.
- For fluid restricted pts: can do IU pitocin as first-line (instead of 166cc 10u/10min initial IV bolus) and then run normal maintenance gtt 5u/hr (30u in 500cc bag is our pharmacy formulary)
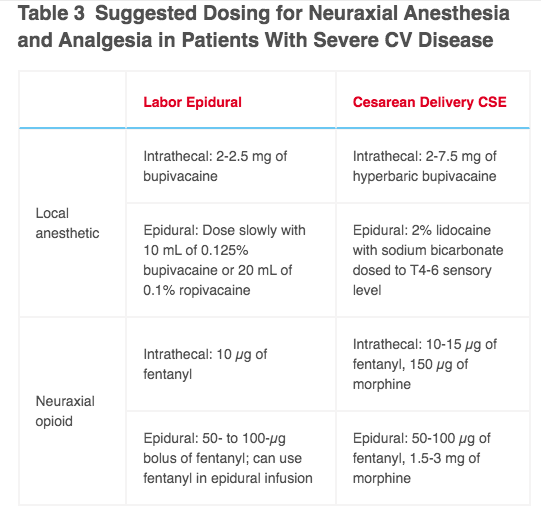
- GA for C/S for emergencies without existing neuraxial, women who require GA/ETT for cardiopulmonary indication, or if neuraxial contraindicated 2/2 therapeutic anticoagulation
- If L&D OR without adequate resources for a complex C/S (eg: space for a TEE machine, ECMO circuit): deliver instead in Main OR, CT, or hybrid OR if peri-partum ECMO is being considered.
- Peri-delivery CO ~ 10 L per minute in healthy women to accommodate this plasma volume shift after delivery.
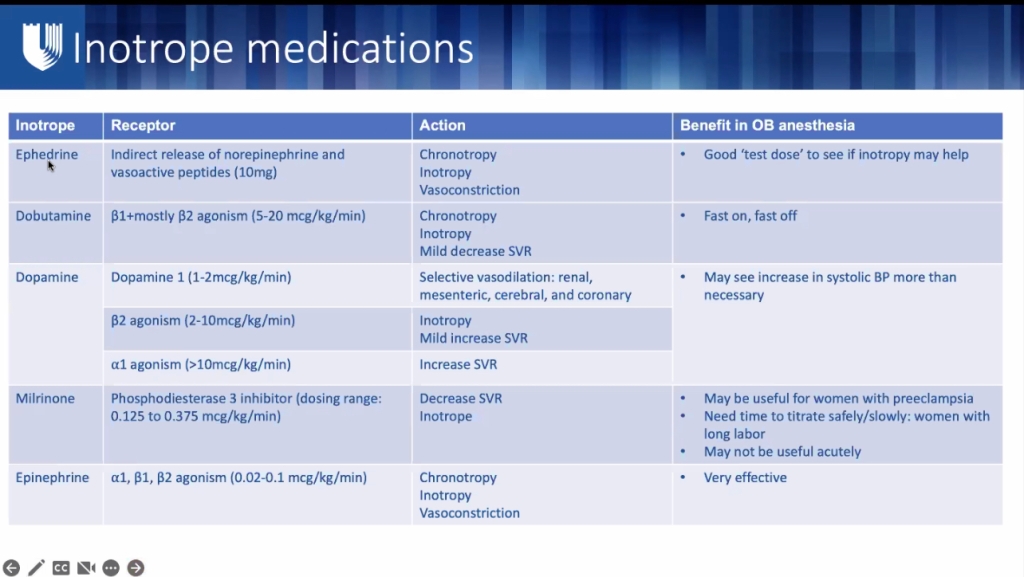
- Consider prophylactic inotropes started at time of delivery to assist myocardium with this sudden increase in workload.
- Arrythmias from inotropes are rare and should not discourage use.
- Generally very effective in these women, even at low doses, as these women are often have naiave receptors to inotropic medications.
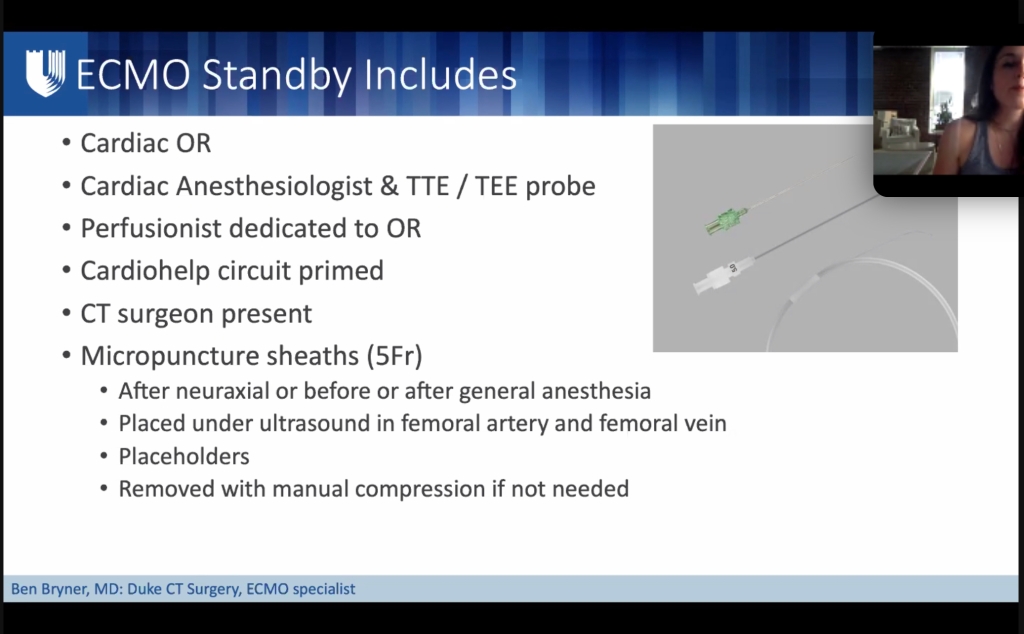
- Any concern that inotrope meds insufficient: (eg: in pulmonary HTN, severely reduced RV function, MPAP pressures >50 mmHg, already on multiple pulmonary vasodilators, or LVEF <20%) consider femoral arterial & venous micropuncture catheters to facilitate emergent ECMO if needed after delivery.
- Micropuncture catheters do not preclude labor and vaginal delivery.
- Consider prophylactic inotropes started at time of delivery to assist myocardium with this sudden increase in workload.
- Hemorrhage Considerations:
- PPH twice as common in women with heart disease.
- The most common cause is uterine atony.
- Higher risk PPH in anticoagulated patients; esp mechanical valves, and CHD, particularly Fontan circulation.
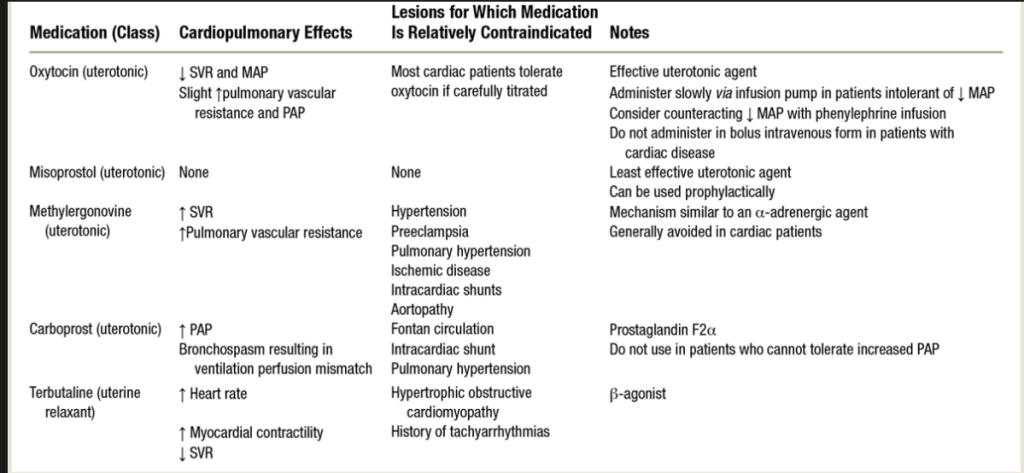
- Uterotonics can have significant HD effects:
- Rapid pit bolus oxytocin can cause vasodilation, HOTN, tachycardia, and myocardial ischemia.
- Pit should be titrated on a pump as bolus dosing can rapidly decrease SVR. Also, pit doses > ED95 (16.2 u/hr in non-laboring C/S, and 44.2 U/h in laboring women undergoing C/S) are not shown to benefit pt, as lower doses are effective and higher doses assc with greater side effects
- Can give as slow 3u bolus or an infusion of 18-36 units/h to avoid side effects, & vasodilation treated easily with vasopressors.
- Carboprost (hemabate) can cause bronchoconstriction and elevated PAP, not given in pt who cannot tolerate an increased PVR.
- shown to increase PVR by > 100% and PAP by 125%. It has been described as precipitating bronchospasm, abnormal V/Q ratios, increased intrapulmonary shunt fraction, hypoxemia, and death. In pts with preexisting asthma, pulm HTN, or right heart compromise, carboprost relatively contraindicated.
- Methylergonovine (methergine) increases smooth muscle cxn via alpha-adrenergic agonism, and can cause HTN, coronary vasospasm, and myocardial ischemia.
- contraindicated in HTN, PreE, aneurysms, or CAD.
- Usually 200mcg IM, but in severe life-threatening PPH, methylergonovine can be diluted and titrated slowly IV to control dose and effect, allowing for antiHTN tx.
Uterotonics and Cardiac Disease:
- Pts at high risk of arrhythmia need continuous ECG during labor & delivery and for 12-to-24 hours postpartum.
- VS Monitoring after delivery will vary by pt condition, but minimum 24 hrs postpartum.
- Multimodal pain control for post-op analgesia
- Intrathecal morphine and scheduled acetaminophen & ketorolac, followed by ibuprofen.
- TAP blocks provide good post-op pain relief for pts w/ contraindication to neuraxial anesthesia.
SOURCE: M.L. Meng MD:
Cardio-Obstetrics: A Review for the Cardiac Anesthesiologist
VALSALVA in CARDIAC Patients
Hemodynamics of a Valsalva Maneuver
- Forceful expiration vs closed glottis, changes occur in intrathoracic pressure that dramatically affect venous return, CO, BP, & HR. This forced expiratory effort is called a Valsalva maneuver.

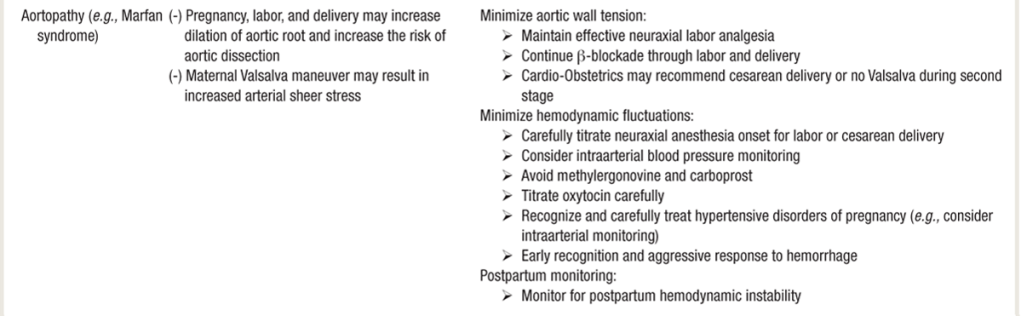
AORTOPATHY
- Aortopathy cannot be allowed to valsalva:
- While holding breath & bearing down, the increased intra-thoracic pressure drops blood return/preload. Then they release breath, and huge preload rush comes back to right heart, pulm vasculature, and left heart – which increases shear stress on aortic root.
- A modified Valsalva maneuver with an open glottis may reduce the changes in intrathoracic pressure that occur with a closed-glottis Valsalva maneuver.
- Heritable fibrillinopathies (connective tissue disorders), bicuspid valve–associated aortopathy, Marfan’s, Ehlers-Danlos, and Turner syndrome are a few of the many causes of aortopathy, which results in aneurysms and dissection in women of childbearing age
- up to 40% all aortic dissections in women < 40y/o are assc with pregnancy
- one of the most common causes of death in pregnancy
- up to 40% all aortic dissections in women < 40y/o are assc with pregnancy
Aortopathy in pregnancy – PubMed
Aorta pathology and pregnancy – PubMed
Marfan’s syndrome and other aortopathies in pregnancy
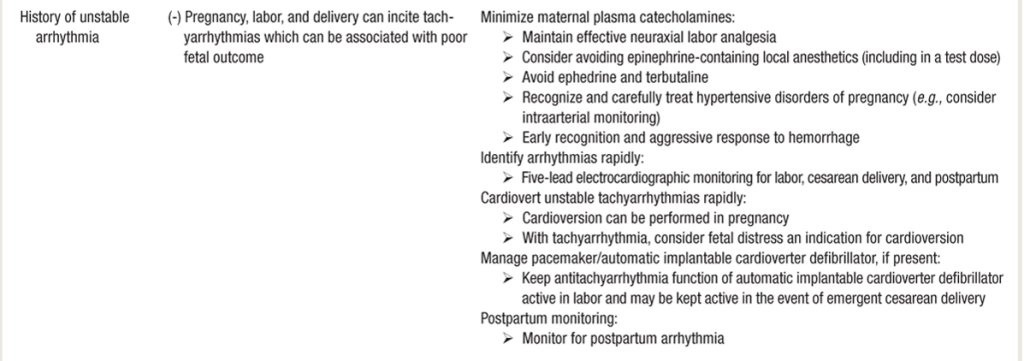
ARRHYTHMIA MANAGMENT in PREGNANCY
- Pregnancy assc w/ increased arrhythmia burden 2/2 cardiac, hormonal, and autonomic changes
- Estradiol & progesterone have been shown to be proarrhythmic in animal studies and case reports of pregnant pts w/ arrhythmias.
- Estrogen shown to increase the # of adrenergic receptors in the myocardium, and adrenergic responsiveness seems to be increased in pregnancy
- Pts w/ hx of arrhythmias have significant recurrence risk
- Pts with structural heart dx & congenital heart disease at higher risk
ECTOPIC beats:
- Very common: 2/2 fluid volume, ANS catecholamines, vagal tone, hormones etc etc:
- PACs, PVCs, usually resolve postpartum/after c.s./after spinal wears off. Unless symptomatic and/or bothersome to mom, usually do not need treatment or workup. If increased vagal tone/visceral traction in c/s causing PVCs – glyco & ephedrine combo can help if needed.
TACHYARRHYTHMIAS:
- Management of tachyarrhythmias depends on the presence of underlying structural heart dx and includes: adenosine, β-blockers, & specific antiarrhythmic drugs w/ safety data during pregnancy.
- Last resort = catheter ablation w/ minimal fluoroscopy (has been done, but preferably wait until postpartum
- Direct Current Cardioversion (DCCV)
- [aka pads on chest & shock: *can sedate w/ ketamine & versed for full stomach & maintained resp – otherwise intubate RSI for the shock – propofol bolus MAC is inappropriate for a full stomach pregnant pt.]
- Very small % of current reaches uterus BUT amniotic fluid = good conductor, so FHR monitor recommended.
- 50-400 J have been used without fetal adverse effects with success rates of >90%. Defib pads placed away from gravid uterus.
- NEED capacity for emergency C/S if needed
- [aka pads on chest & shock: *can sedate w/ ketamine & versed for full stomach & maintained resp – otherwise intubate RSI for the shock – propofol bolus MAC is inappropriate for a full stomach pregnant pt.]
SVT
- Among most common
- 1st line: vagal maneuvers 1st, then A
BC: ADENOSINE, BB, CCBS- Adenosine 6mg, followed by 12mg if unsuccessful)
- USE BB 1st instead in adenosine or CCB in WPW – adenosine and CCBs (AV nodal blocking drugs) can worsen arrhythmia via increased conduction down accessory pathway worsening maternal condition.
- If adenosine fails (1st line ~90% effective)**, use βBs (2nd line: metop* cardioselective) or CCB (verapamil up to 10mg 3rd line) used for acute termination of AVNRT w/ known pre-excitation/ hx paroxysmal SVT. *Close monitoring for conversion of AVNRT to pre-excited AF.
- These meds can ^^conduction over accessory pathway, ^^ pt risk of AF degenerating into ventricular arrhythmias.**
- Adenosine can also send pt into PRE-TERM LABOR [induces uterine cxns] – make sure OBGYN available (tocolysis can be achieved with CCBs)
- Adenosine can cause bronchospasm in asthmatics – can give albuterol to prevent/treat, but a B2 agonist isn’t doing us any favors when treating a tachyarrhythmia… If albuterol needed, you could theoretically give selective B1 blocker (esmolol or metoprolol) to block HR response.. Otherwise could just start with CCB verapamil 5mg IVP over 2 min, dose up to 10 mg.. and move on to adenosine if Verapamil fails.
- If adenosine fails:
- BB metoprolol 2nd line (1st line tx in WPW)
- 5mg over 2 min slow IV push, repeat Q5 min up to 15mg IV to HR goal < 110.
- Atenolol contraindicated in 1st TM*
- CCB Verapamil 3rd line
- Contraindicated in 1st TM
- (dose: 5mg IV over 2 min slow IV push, up to 10mg IV) is OK. Verapamil OK in 2nd and 3rd TM (fetal teratogenicity in 1st TM)
- (Diltiazem not advised (while both are the same class of CCB non-dihydropyridines) – Dilt is controversial – fetal adverse effects in animal models)
- Digoxin (class: digitalis cardiac glycoside) also safe – can be combined with adenosine and BB for treatment.
- Amiodarone reserved for refractory cases and life-threatening cases, used in conjuction with Cardioversion.
- BB metoprolol 2nd line (1st line tx in WPW)
- If adenosine fails:
- Adenosine 6mg, followed by 12mg if unsuccessful)
- REFRACTORY SVT CAN BE CARDIOVERTED IN ANY TRIMESTER FOR UNSTABLE MOM!!! Charge: 50-400J biphasic
Stanford Emergency Manual guidelines, with diltiazem switched for Verapamil in pregnancy:

Supraventricular Tachycardia in Pregnancy: Gestational and Labor Differences in Treatment
Verapamil in SVT: No Need to Drop the Beat
A-FIB/A-FLUTTER
- More likely with congenital, valvular (esp mitral), & ischemic heart disease, common onset in 2nd TM
- As low as 1.3%, but as high as 39% incidence with more severe CV dx.
- Pregnancy = hypercoagulabe state = consider anticoagulation status if neuraxial needed.
- UNKNOWN onset ? – get Trans Esophageal Echo: TEE to eval for thrombus before DCCV.. (requires ETT in pregnant full stomach)
- NEW ONSET AF/Aflutter: get Trans THORACIC echo: TTE eval for structural heart disease (no anesthesia needed, onset time too soon for thrombus development)
- Rule out: thyroid dx, electrolytes, PE, & ETOH abuse
- 1st line: BB for rate control (metop cardioselective)
- 5mg over 2 min slow IV push, repeat Q5 min up to 15mg IV to HR goal < 110.
- 2nd line: CCBs: Verapamil 5mg over 2 min, up to 10mg
- 3rd Line: Amiodarone as LAST RESORT: for refractory, unstable Afib and needed along with DCCV: 150mg loading dose, then gtt 1mg/min. (reserved for emergency cases)
- CARDIOVERSION: DCCV if refractory and if any signs of instability develop. DCCV should be done anyway within 48h to minimize stroke risk, even if rate control is pharmacologically achieved.
- Can do catheter ablation in refractory cases, but best to wait until postpartum.
- *Mitral stenosis should be fully anticoagulated [careful timing of neuraxial if anticoagulation able to be stopped]
V-TACH
- Rare except in congenital heart dx: risk ~ 27%
- Tx tailored to underlying heart dx/structure
- 1st line: Lidocaine & βBs safe (in stable pt).
- No Class IC agents if VT 2/2 CAD or myocardial ischemia
- IF UNSTABLE: EMERGENT DCCV (bc maternal instability = high risk fetal compromise)
- DCCV at 50-100J (higher energies at 100-360J if needed)
- Amiodarone if life-threatening and refractory (300mg, then 150mg)
- MgSO4– 1-2g IV in Torsades or polymorphic VT

Sources:
Supraventricular Tachycardia in Pregnancy: Gestational and Labor Differences in Treatment – PMC
2023 HRS expert consensus statement on the management of arrhythmias during pregnancy
Contemporary Management of Arrhythmias During Pregnancy
Managing palpitations and arrhythmias during pregnancy
Management of Cardiac Arrhythmias During Pregnancy
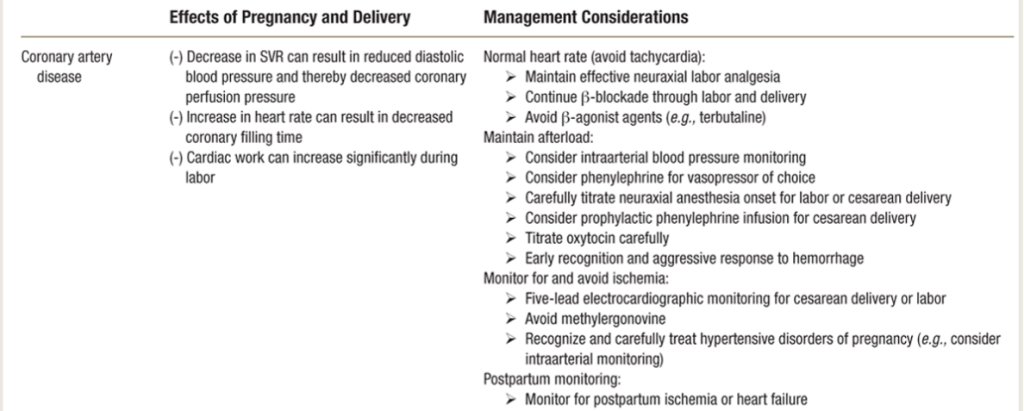
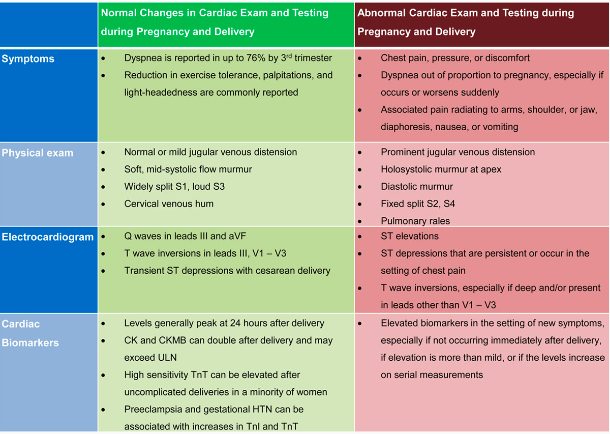
Ischemic Heart Disease/SCAD/MI in Pregnancy.
- Risk of acute MI is 3- to 4-fold higher in pregnancy.
- 2.8 – 8.1 per 100K deliveries; mortality 4.5% to 7.3%
- Pre-existing CAD: 10% of pts have ischemic complications.
- Pregnancy-related SCAD (spontaneous coronary artery dissection) & MI with non-obstructive coronary arteries are prevalent causes.
- Pts with hx SCAD, or hx CAD/IHD ARE VERY HIGH RISK and may have LV dysfunction & signs of residual ischemia.
- Atherosclerosis CAD <50% of patients (bc usually younger healthier women)
- 3rd TM & postpartum are the highest-risk periods for cardiac OB pts.
- Pregnancy-assc MI (PAMI) = > 20% of maternal cardiac deaths, risk continues through 12 wks postpartum as cardiac changes or pregnancy return to baseline.
- Nearly half of maternal deaths occur within the first day of delivery, and 66% of deaths occur within the first week.
- Hypercoagulability decreases by 6 wks and normalizes ~ 12 wks.
- Mechanical prosthetic heart valves assc with increased risk of fetal and maternal morbidity and mortality
- Maternal risks: increased mortality, valve thrombosis–associated valvular dysfunction, heart failure, stroke, and maternal hemorrhage.
- Fetal risks: increased mortality, teratogenicity, and hemorrhage
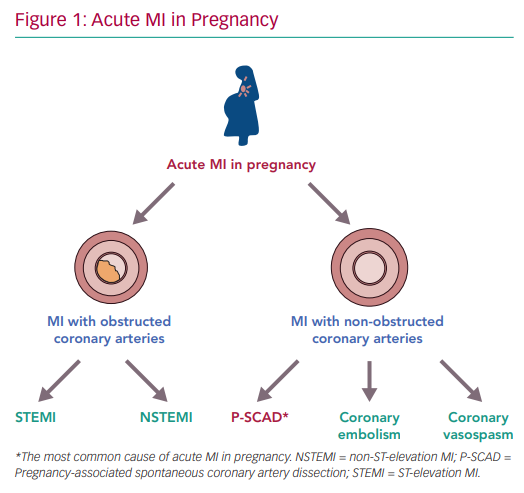
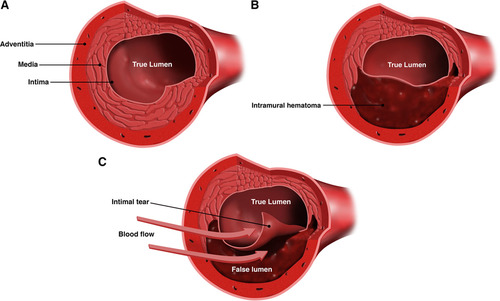
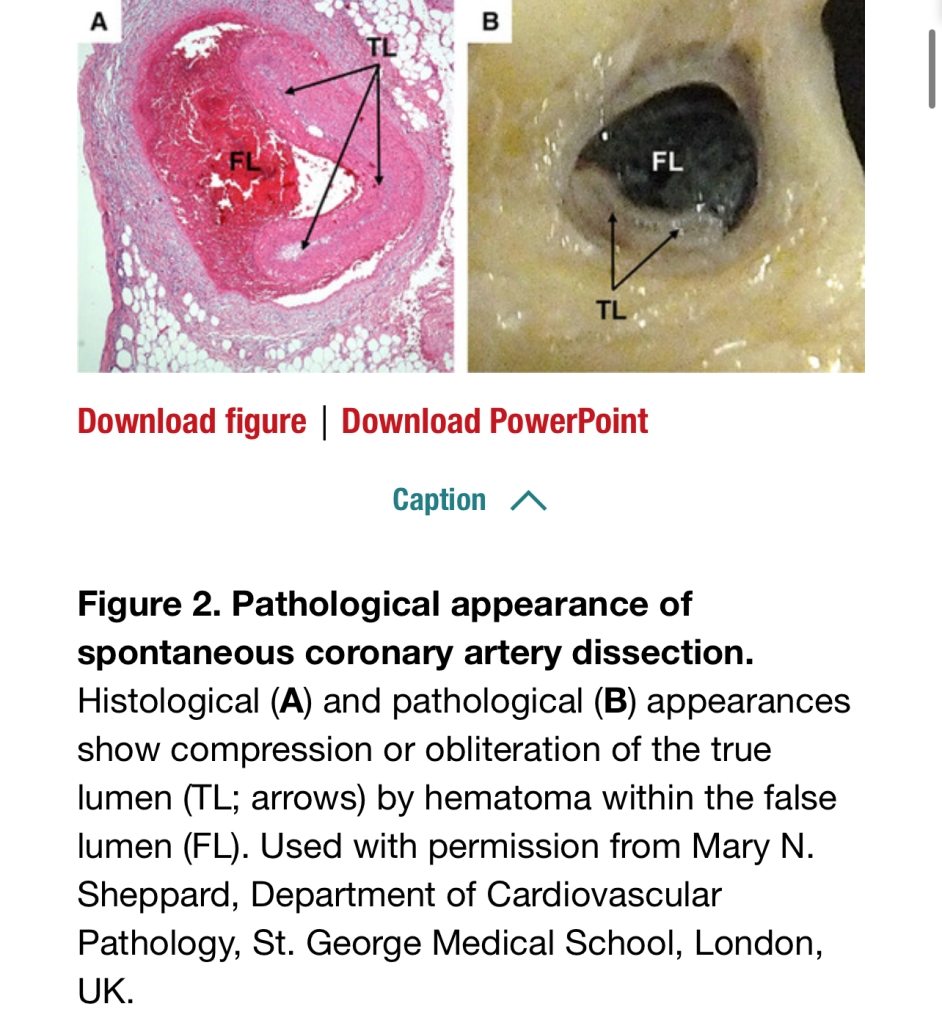
SCAD
- Two theories: 1) Intimal tear in coronary artery created false lumen which fills with blood and obstructs true lumen. 2) Spontaneous hemorrhage in vasa vasorum of artery wall.
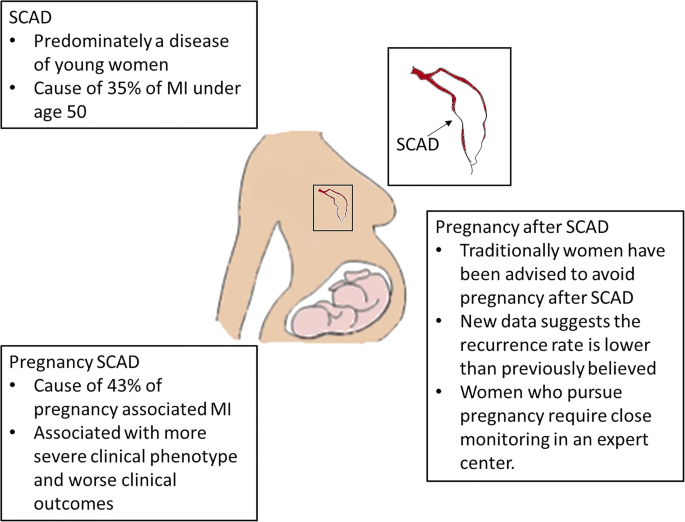
- Often occurs in women with NO CARDAC HX or RISK FACTORS (HTN, DM, HLD)
- may account for 1-4% ACS overall, and up to 35% ACS in women < 50y/o
- Predominantly affects women of childbearing age, and is a leading cause of ACS in pregnancy.
- highest risk 3rdTM through 6 wks postpartum
- suggests multifactorial etiology influenced by hormones, genetics, underlying atereriopathies, systemic inflammation, and possible environmental factors & stressors.
- Presents as STEMI or NSTEMI. Can often resolve with conservative TX.
- S/S: chest pain most common, arrhythmias, Elevated cardiac biomarkers
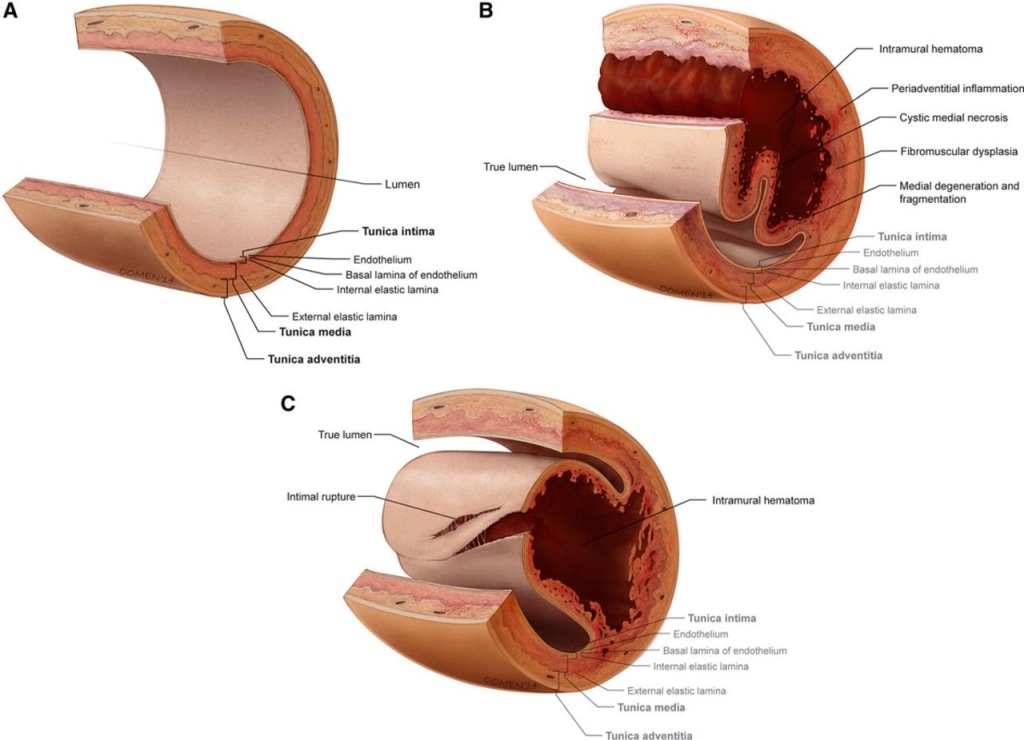
SCAD Symptoms:

MANAGEMENT ALGORITHMS
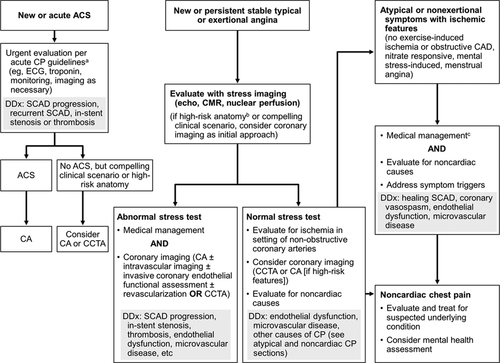
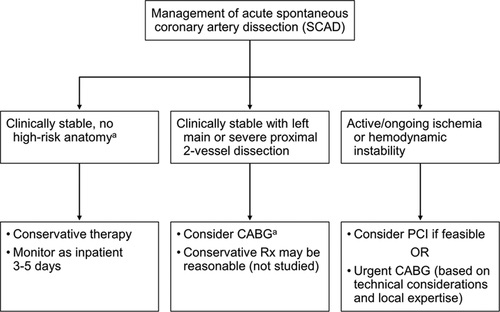
ACUTE MI: WHAT DO WE DO IN THE OR/LABOR ROOM?!
- If mom is unconscious – intubate and support with ACLS & STAT delivery.
- If mom is awake & conscious with STEMI/NSTEMI:
UPDATE:
- (Our girl MONA has been debunked) r.i.p MONA
- Morphine: may be assc w/ delayed & decreased GI absorption of anti-PLTs, LARGER infarct size, & increased mortality.
- Oxygen: in NON-HYPOXIC pts: May INCREASE infacrt size, contribute to arrhythmias and not reduce pain.
- Nitrates: (although still a mainstay tx) nitroglycerin may cause endothelial dysfunction and have toxic pro-oxidant effects. mixed data.
- ASPIRIN is only agent with clear proven benefits. Suspected Acute MI pts can chew a 325mg tablet for fastest onset of PLT inhibition.
SO WHAT DO WE DO ?
- Defib pads on
- 12-lead ECG
- Fentanyl small doses can be given for ++pain
- Aspirin 325mg chewable
- O2 only if SpO2 < ~94%, minimum FiO2 for adequate maternal oxygenation, while maintaining fetal oxygenation, which decreases on fetal hgb dissociation curve around maternal PaO2 of 70 or below
- Draw labs: chem, CBC, troponin, CKMB
- PCI IS DEFINITIVE TREATMENT in unstable patient.
- Pt needs Angiograpy +/- reperfusion therapy.
- Possible exception is SCAD.
- PCI in SCAD reserved for left main, continued chest pain/ongoing ischemia, & instability
- radial forces from balloon/stent expansion may broaden dissection.
- high rate of lesion recovery within months. yay!
- antiplatelet agents combined with β-blockers (ie, labetalol) for conservative tx.
- PCI in SCAD reserved for left main, continued chest pain/ongoing ischemia, & instability
Cardiac Biomarkers
- TROPONIN is SPECIFIC TO MI in pregnancy, while CK MB is not.
- Elevated Troponin ABNORMAL in pregnancy
- Elevated CKMB is NORMAL in pregnancy
- Elevated Troponin:
- Mild troponin elevations may be seen in PreE & gHTN
- But Elevated Troponin1 in pt without a diagnosis of pregnancy ‐assc HTN = PRIMARY CORONARY EVENT (> 0.04 ng/mL)
- Creatine Kinase Myocardial Band (CK MB):
- is normally present in the uterus & placenta
- CKMB rises and can quadruple for 24 hours s/p delivery, then declines.
- CKMB less specific for dx Acute MI during pregnancy, but is still used as an addition to other lab tests for a complete picture.
Obstetric Approach to STEMI in Pregnancy.
- Vaginal spontaneous delivery preferred; but case by case.
- IOL with pitocin can be planned to allow for planned d/c of antithrombin therapy /DAPT before delivery
- SPONTANEOUS non-induced vaginal delivery BEST
- Avoid CV effects of pitocin, and fluid balance monitored.
- ST‐depression, ischemia, arrhythmias have been assc w/ oxytocin gtt/ boluses
- Synthetic prostaglandin analogs = risk of coronary vasospasm & arrhythmias (CARBOPROST/hemabate, & MISOPROSTOL/cytotec)
- Avoid CV effects of pitocin, and fluid balance monitored.
- In athlerosclerosis-related STEMI –> PCI is treatment.
- In coronary artery dissection (SCAD), conservative tx OK for stable pts without ongoing pain.
- Pts w/ chest pain, ischemia, ST-elevation, HD instability, dissected left main, or causing myocardial jeopardy – IF willing to have to PCI, should undergo percutaneous revascularization.
- Uterus can be covered in lead for PCI. Risk to fetus is minimal.
Anesthesia Considerations
- Neuraxial with Epidural for labor or CSE for C/S generally preferred.
- HOTN minimized with lower‐dose LA + vasoconstrictors/IVF
Major NEURAXIAL hematoma concern in cardiac pts receiving DAPT.
- ASRA COAGS :
- ASRA says d/c Clopidogrel 5 – 7 days before neuraxial.
- *ASPIRIN ALONE* IS OK for Neuraxial
- UFH and LMWH must be D/Ced (24 h for therapeutic LMWH)
- Multidisciplinary approach to determine when DAPT should be re-started in the postpartum period. Risk epidural hematoma vs risk thrombosis.


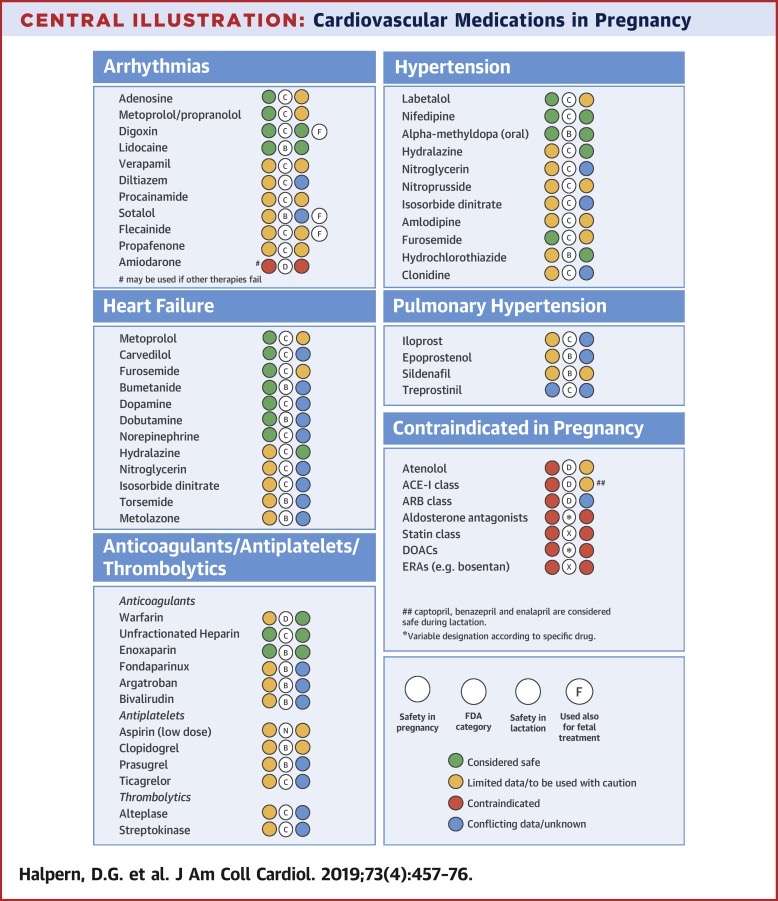
Ischaemic heart disease in pregnancy
114. Cardio-Obstetrics: Pregnancy and Coronary Disease with Dr. Malissa Wood
Cardiac Disease or Preeclampsia? An Unusual Case Study
Cardiomyopathy & Stenotic Lesions in Pregnancy


- SOAP 2019 Maternal Cardiac Disease Delivery Planning Algorithm/Framework
- Marie-Louise Meng, MD & Katherine Arendt, MD

Review of Non-Valvular Heart Disease in Pregnancy:
SOAP OB Maternal Critical Care Lecture Series 2022
- 10 total Maternal critical care lectures
- Multi-disciniplinary: OB/MFM/ICU/OB Anes.
- Fellow-Level.
SOAP WEBINAR JUNE 2022 TITLE SLIDE:

LINK:
MC LMS – 2022 Webinars – SOAP June 2022 Maternal Critical Care Series Webinar
June 2022 LECTURE NOTES:
WHY is Heart Disease and CM such a HUGE topic in OB lately?
- Pre-existing heart disease carries 100-fold increased risk of maternal death.
- Maternal death rates on the rise in US over past 30 years
- leading causes of pregnancy mortality are cardiovascular conditions and CM
- Disproportionately affects black non-hispanic women: systemic racism & lack of access to appropriate care.
- More and more pedi congenital heart disease pts are living into adulthood and having children. All cardiac defects are at higher risk for CM. even ASDs.

- ^^ In order: CV conditions, infection/sepsis, Cardiomyopathy, hemorrhage, CVA, HTN disorders of pregnancy, AFE, Anesthesia complication (failed airway)
- Other non-CV medical conditions

^^ Pregnancy-related deaths per 100,000 live births 1987-2017
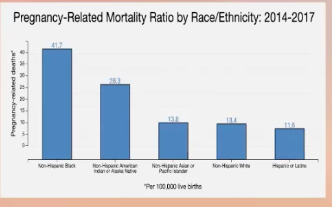
^^ From 2014-2017, per 100,000 live births: Non-Hispanic Black women had highest rate of pregnancy-related death (41.7), followed by Non-Hispanic American Indian or Alaskan Native (28.3), Non-Hispanic Asian or Pacific Islander (13.8), Non-Hispanic White (13.4), and Hispanic or Latino (11.8)
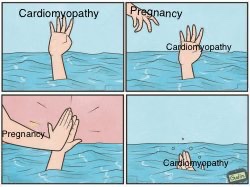
SEVERE CARDIAC DISEASE IS A CONTRAINDICATION TO PREGNANCY, yet we still see these patients.
- lack of prenatal care
- health education barriers
Normal cardiac changes in pregnancy that are especially concerning in CARDIOMYOPATHY: Increased Chamber size, & Increased Plasma Volume
Normal cardiac changes in pregnancy that are especially concerning in STENOTIC LESIONS: ^^ HR, ^^BP, ^^CO
Normal Physiologic Changes in Pregnancy table:
CARDIOMYOPATHY
3 main types:
- Dilated CM (DCM)
- HOCM
- Peripartum CM (PPCM)
Causes of Cardiomyopathy:
- Idiopathic ~ 50% (esp young pts)
- Genetic ~ 40%
- Inborn errors of metabolism (IEM) ~7%
CM patients: ECHO capability ESSENTIAL.
- TTE Portable US/POCUS, must be available for these pts to monitor acute HD changes PRE AND POST-partum.
Dilated CM:
The usual Goal-directed med therapy:
- MEDS: ACE-I, Spironolactone, BB, Diuretic, inotropes (milrinone, dopamine)
- Pregnant pt would have to stop ACE-I & Spinonolactore during pregnancy, so may not be as well controlled coming into pregnancy, then compounded by massive HD volume changes of pregnancy—> deterioration/acute HF.
- highest risk of cardiac events in 3rd TM AND postpartum (most profound CV changes & dysfunction)
- risk of fetal adverse events: preteen delivery and FGR.
- CV risk continues in postpartum period, and CV risk can last long-term


PERIPARTUM CM (PPCM):
PPCM UPDATE: NEJM January 2024
PPCM
- EF< 45%, new onset without prior cause/cardiac hx – dx by exclusion.
- USA stats: “1:4,000” but more like 1:1,000 in the South
- > 40y/o, Black, from Southern states seems to be highest prevalence.
- Wide international variation; eg) parts of Haiti (1:300) and of Nigeria (1:100)
- Cause: definitively-unknown, diagnosis by exclusion, some cases may have genetic contribution.
- TX: maybe Bromocriptine 2/2 prolactin suppression (dopamine D2 agonist – inhibits pituitary prolactin secretion). Prolactin recently found to have relationship to heart disease and dysfunction in pregnancy.
- Otherwise supportive management, inotropes, diuretics, etc.
- Mainstays of HF are ACE-Is and Spironolactone – both contraindicated in pregnancy, so pts with existing HF may be under-optimized prior to delivery.
- Otherwise supportive management, inotropes, diuretics, etc.
- May need Anticoagulation d/t risk of LV thrombus from low LVEF.
- If PPCM presents and EF never normalizes afterward (usually normalizes within a year postpartum), or EF < 30%, subsequent pregnancies at much higher risk for severe PPCM: mWHO CLASS 4: pregnancy contraindicated)
Proposed pathobiology of PPCM
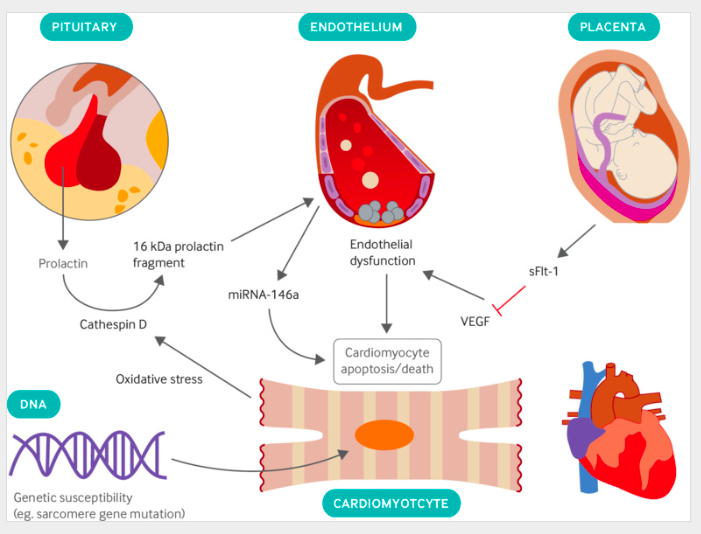
Secretion of prolactin by the anterior pituitary gland, upregulation of endothelial microRNA-146a (miRNA-146a), and placental secretion of soluble fms-like tyrosine kinase receptor 1 (sFlt-1) lead to endothelial dysfunction and cardiomyocyte death; genetic susceptibility is also present in some patients. (VEGF=vascular endothelial growth factor.)
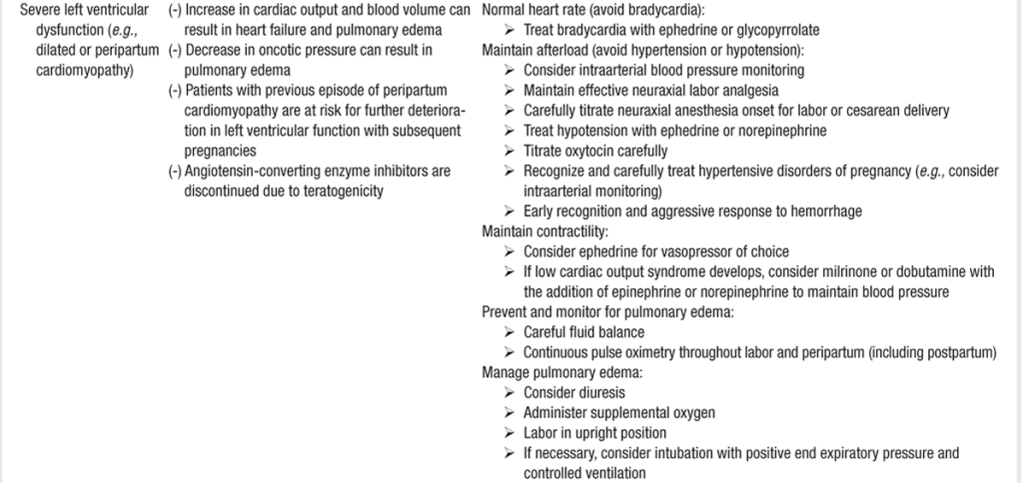
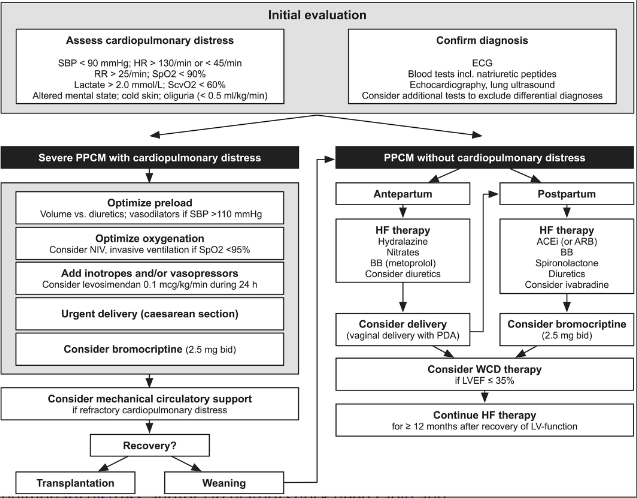
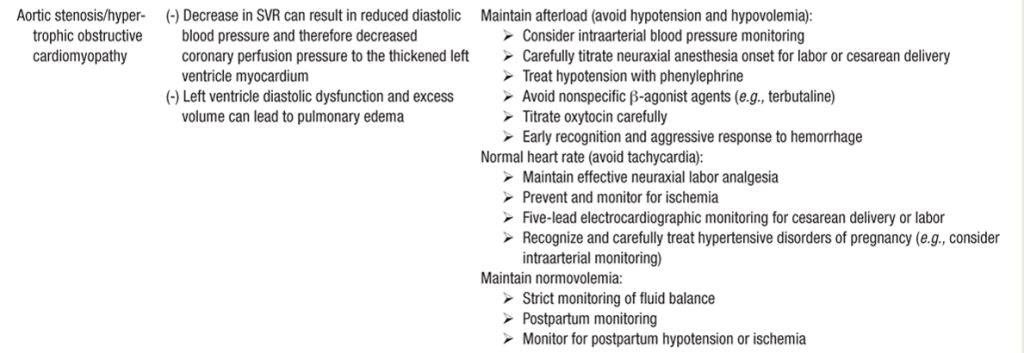
Anesthesia for Cardiomyopathy
- Similar to Dilated CM anesthesia guidelines listed in previous section.
- Vaginal delivery preferred if not super severe & complicated.
- Should have invasive monitor (A-line) and 5-lead EKG
- Early epidural can help blunt tachycardia with cxns/pain/SNS surge.
- Caution with decreased SVR & preload assc w/ neuraxial.
- Slow, gentle onset epidural. **esp c/HOCM.
- Dobutamie first line inotrope, norepi for shock
- Optimization of preload: IVF vs diuretics.
- If no sign of overt fluid overload, fluid challenge (250–500 mL over 15–30 min) is recommended, especially in pts with intravascular depletion 2/2 peripartum blood loss or overaggressive diuretic therapy.
- If congestion present , intravenous diuretics should be administered. (POCUS lung US)
- In pts with SBP >110 mmHg, intravenous vasodilators (e.g. nitrates) should be started.
- SpO2 maintained > 95%. NIV preferred unless AMS or persistent hypoxia requires ETT.
Peripartum TTE
- antepartum (compare to baseline)
- intrapartum (monitor epidural effects, labor autotransfsion~500cc from each normal labor cxn)
- postpartum – within 30 min of delivery (auto transfusion from uterus contracting down)
- again PPD #1, and if PPH.
Episodes to listen to:
Peripartum Cardiomyopathy – The Fundamentals
Peripartum Cardiomyopathy Part 1: Presentation and Diagnosis
Peripartum Cardiomyopathy Part 2: Labor and Birth
Peripartum Cardiomyopathy: Definitions, Diagnosis and Management
Peripartum cardiomyopathy | The BMJ
HOCM
- Want slow NSR <100 bpm, preferable < 80 bpm.
- Heart needs time to fill in diastole to maintain SV and C.O.
- Thickened heart muscle needs diastolic time to allow for coronary perfusion of thickened muscle
- Slow NSR maintains myocardial supply/demand balance, as well as giving sufficient time for diastolic filling in less-compliant left ventricle.
- especially in pregnancy where O2 demand and consumption is much higher than non-pregnant state.
- Decreased coronary perfusion of thick muscle will led to dysrhythmias
- HOCM still generally well-tolerated in pregnancy, esp if no pre-pregnancy s/s.
- Mortality 0.5%, & ^ risk preterm delivery
- HOCM pts can generally can do OK with vaginal delivery.
Findings in HOCM:
- Thickened septum +/- Systolic Anterior Motion (SAM) of anterior mitral valve leaflet
- Dynamic LVOT causes flow acceleration/turbulence (coanda) through aortic valve.
- LVOT obstruction worsens with decreased LV preload, and VALSALVA (ie: pushing – decreases preload – explained way further down in this page)
- **Can be misdiagnosed in young pts (genetic HOCM) as “exercise-induced asthma” **
Vaginal Delivery with epidural OK, but needs SLOW, GENTLE onset epidural block. Decreased SVR & preload from sympathectomy & vasodilation will worsen LVOT.
Meds for HOCM:
- BB if > mild LVOT (mod-severe >15mm septal thickening)
- NEED SLOW SINUS rhythm, no tachycardia or Vent arrhythmias.
- complications from arrhythmias —> HF
- High risk HF postpartum 2/2 immediate post-delivery auto-transfusion & CO doubles x 48h. Takes 2 weeks to return to pre-pregnancy baseline.
- May need postpartum cardiac surgery to fix HOCM.
HOCM Maternal Adverse Cardiac Events (MACE)
- 1st TM: HF & Vent Tachy Arrhythmia
- 2nd TM: HF & A-fib
- 3rd TM: HF and VTA highest risk
- Postpartum: HF highest risk


3. Hypertrophic Cardiomyopathy: Case Discussion
VALVULAR LESIONS:
Valvular heart disease accounts for approximately 15% of cardiac disease in parturients, with rheumatic disease being most prevalent. Stenotic abnormalities carry highest risk for both the mother and fetus. Regurgitant lesions are better tolerated, but can worsen to the point of increasing the NYHA class.
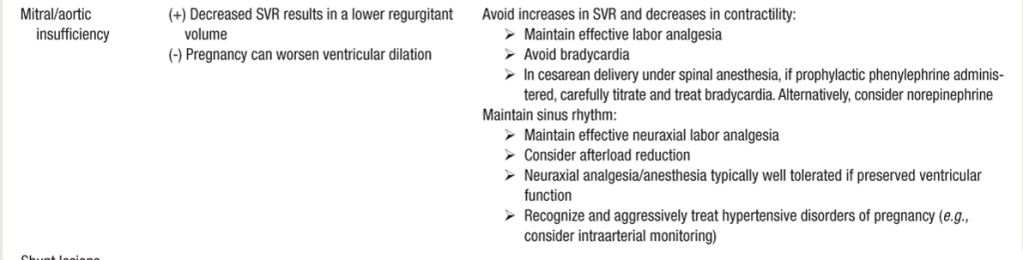
- Regurgitant lesions generally well-tolerated in pregnancy. Can also be a new pathological finding in pregnancy from fluid overload.
- Regurgitant Left-heart lesions: “FULL, FAST, AND FORWARD”
- FULL: adequate preload (SV) maintains adequate EF%,
- FAST: HR x SV = CO: EF% may be only a small fraction of EDSV since part of EDSV goes backwards through imcompetent valve. IF SV is already small, a low HR will dramatically reduce CO.
- FORWARD: Avoid increases in SVR (HTN), which will only encourage backward flow. LA can then become overloaded and place pt at risk of pulm edema. Ephedrine may be better for BP augmentation, as added beta-agonism contractility will help overcome SVR in increase forward flow. Can see paradoxical decrease in HR after ephedrine administered, as SV/EF improves, and HR able to decreases as a function of restored CO.
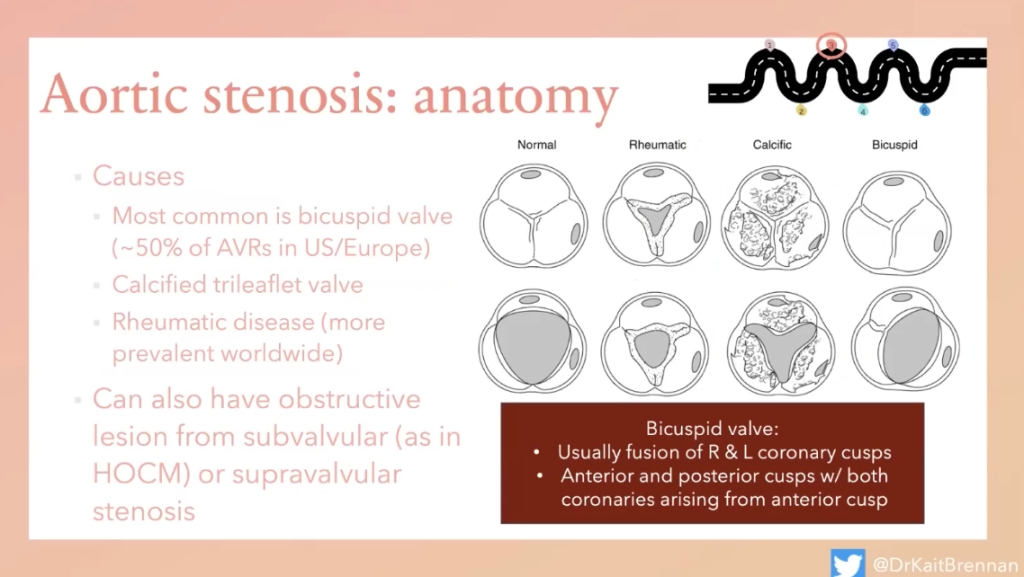
AORTIC STENOSIS (AS)
Bicuspid vs. rheumatic vs. calcified tri-leaflet
- Bicuspid aortic valve: common to have aortic root dilation as well.
- AS: LV afterload —> compensatory LVH —> vent remodeling —> poor diastolic filling —> relies on NSR atrial kick(active filling), —> poorly tolerates any arrhythmias/non-sinus rhythms.
- RISK OF SUBENDOCARDIAL ISCHEMIA in any low BP state.
- higher LDVEP = dec. myocardial perfusion pressure (LV perfused during diastole)
- *esp during induction of GA or placement of neuraxial (SVR drop)
- Risk of maternal cardiac complications: HF (10-25%) & arrhythmia 3-10%
- Higher risk of c/s, preterm delivery, and FGR.
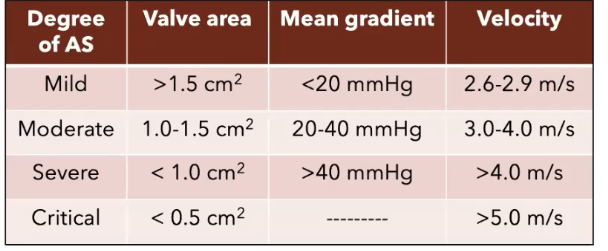
AS in Pregnancy
- Most common complications are HF(10-25%) and arrhythmias (3-10%)
- More common complications if AS severe
- Higher rate of c/s, preterm delivery(severe AS), and FGR
Anesthetic Management for AS in Pregnancy
- Ensure slow, NSR
- Invasive BP monitor – A-line for moderate & severe AS.
- AS death spiral: HOTN—>dec coronary perfusion pressure—>subendocardial ischemia—MI
- Phenylephrine is best pressor. Alpha only. Keep afterload high without stressing myocardium /increasing myocardial O2 demand w inotropes/beta stimulation. Give phenylephrine prophylactically if BP drop expected.
- Caution w/ sympathectomy in epidural. (Spinal is inappropriate – neuraxial for c/s should be slow-dosed epidural)
MITRAL STENOSIS
[Goal: *UNLOAD, and SLOWED*: BB tx to NSR, keep dry/decreased preload]
- MS = 12% of all valvular lesions (in US less common, but in developing world much more common 2/2 childhood rheumatic fever. Global decline 2/2 improved tx of streptococcus. MS has a slow progression of valve calcification and stiffening over 20 – 30 years.
- Mitral Valve Area (MVA) impedes LV filing —> ^^LA pressure, LA enlargement —> Increased pulmonary venous & arterial pressures (pulm HTN), and eventually leads to R-sided heart failure often w/ assc tricuspid valvular insufficiency.
- The smaller the MVA, the higher the LA Pressure
- S/S severe MS: chest pain, dyspnea, palpitations, pulmonary edema, hemoptysis, and thromboembolism.
- Assessment of disease severity should include:
- LA dilation
- Mitral regurgitation
- pressure gradients
- RVSP (measure of Pulm HTN – function of back-pressure from LA),
- MS gradients are much less than AS gradients bc this is coming from an atria which is generally a low pressure system, whereas AS is coming from the left ventricle (high pressure.)

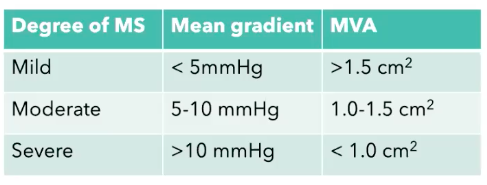
Mitral stenosis (mod-severe) poorly tolerated in pregnancy, due to:
- ^^ Circulating vol (Plasma vol increases ~40% by 28wks, ~50% by 35wks)
- can decompensate into cardiogenic shock/pulm edema
- ^^ CO in 2nd & 3rd TM 2/2 plasma volume increases
- ^^ HR of pregnancy
- Some pts OK for balloon valvuloplasty in 2nd TM
- Can expect early delivery
- Severe MS has improved outcomes w/ intervention prior to pregnancy:
- surgical mitral valve commissurotomy, valve replacement, or percutaneous mitral commissurotomy.
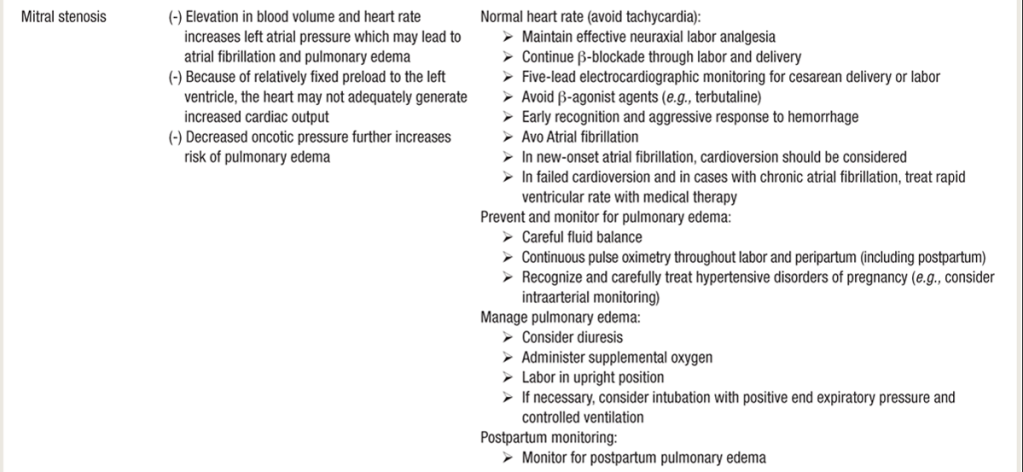
ANESTHETIC MANAGEMENT of MS in Pregnancy
- For severe lesions: involve CARDIAC anesthesia & best-case in cardiac OR
- 5-lead EKG and A-line
- Risk of Afib = ANTICOAGULANTS = neuraxial placement & dose timing
- Beta blockers (want NSR). Tachycardia = less diastolic falling time for LV, & very poorly tolerated.
- DIURESIS: Prophylactic DIURESES – keep them as dry as is safe (dilated LA —> pulm edema)
- Vaginal delivery: early pain control with IV opioids or epidural analgesia.
- 2nd stage of labor (10cm, pushing & delivery) increases preload and CO as a result of autotransfusion from the uterus and relief of aortocaval compression.
- These alterations in hemodynamics and cardiac preload can cause acute pulmonary edema.
- Labor CSE technique, where intrathecal opiates given early in the first stage of labor followed by gradual titration of dilute epidural LA as labor progresses to 2nd stage (to cover T10–S4), provides HD stability for most pts.19
- 2nd stage of labor (10cm, pushing & delivery) increases preload and CO as a result of autotransfusion from the uterus and relief of aortocaval compression.
- If C/S: slow-dosed epidural best if time allows.
- “Single-shot” spinal avoided in both mod – severe MS as abrupt changes in SVR are poorly tolerated.
- No epi in test dose for risk of tachycardia/arrhythmia. 50 mcg fent is an adequate test dose.
- Slow epidural titration of 2% lido combined with fent for T4–T6 surgical level. PRN titration of small 50 – 100μg neo will counteract the expected HOTN from sympathectomy.
- SBP ideally > 100 mm Hg, although may possibly exacerbate pulm HTN.
- If echo in 2nd or 3rd TM trimester suggests pulm HTN or right heart “overload” (i.e., RV/RA dilation, often w/ tricuspid regurgitation), then consider pulm artery catheter (PAC) for labor and delivery.
- Avoid meds causing tachycardia:
- glyco, atropine, meperidine, pancuronium, ketamine, beta-agonists: albuterol (unless necessary), terbutaline.
- N2O increases PVR – may aggravate RV dysfunction
- IF Spinal wears off and Epidural re-dose FAILS:
- (pain also causes tachycardia) Still arguably better to just use small doses of versed & ketamine (and glyco if secretions worrisome) and control HR with Esmolol/BBs. Converting to GA would also require BBs to blunt stimulation of intubation, so ether way, we’re beta blocking, and ETT/GA airway instrumentation is less safe than maintaining spontaneous resp, plus the N20 we usually use under GA to reduce Sevo% (uterine tone) can increase PVR.
- While IV Fentanyl does not cause tachycardia, the large IV doses required would likely cause respiratory depression/hypoventilation (intrathecal narcotics in place too) and IV fent is generally not used to manage pain of failing neuraxial for these reasons. Ketamine has much more profound analgesic properties while maintaining respiratory drive.
- Vaginal delivery: early pain control with IV opioids or epidural analgesia.
- The blood loss (volume reduction) in vaginal delivery & CD are actually beneficial to MS pt, as long as adequate cardiac preload maintained.
- Tachycardia decreases LV diastolic filling time through the stenotic valve.
- If GA used: reduce sympathetic response to DL:
- Remifentanil (0.5 to 1 μg/kg) and a short-acting β-adrenergic blocker such as esmolol (30 to 50 μg/kg).
- Etomidate to maintain preload/SVR, & succs RSI.
- Avoid meds causing tachycardia:
- glyco, atropine, meperidine, pancuronium, ketamine, beta-agonists: albuterol (unless necessary), terbutaline.
- N2O increases PVR – may aggravate RV dusfunction
- If needed, GA allows for TEE to assess need for +/- fluids, inotropes, and vasopressors, or lasix.
- If GA used: reduce sympathetic response to DL:
- Aggressive maintenance of NSR and management of A-fib (2/2 LA dilation)
- detrimental bc atrial “kick” ~ 30% of the LV stroke vol in MS pts.
- β-blockers agents, unless contraindicated to NSR ~ HR < 90
- A-fib also means pt may need anticoagulation (check for neuraxial)
- detrimental bc atrial “kick” ~ 30% of the LV stroke vol in MS pts.
- NEURAXIAL IS OK – slow dose epidural
- “modified test dose” – don’t want epi going IV and causing tachycardia
- Either: Lido only – looking for metallic taste in mouth /ringing in ears, OR 50 mcg fent
- “modified test dose” – don’t want epi going IV and causing tachycardia
- Avoid additional increases in PVR (back pressure from LA)
- adequate pain control, avoid hypoxia, hypercarbia, & acidosis. Can give supplemental O2
- HIGH RISK DECOMPENSATION from AUTOTRANSFUSION post-delivery. Decompensation from LA not functioning/able to overcome stenotic MV, —> pulmonary edema —> intubation.

Tricuspid Stenosis
- Very rare, (normal valve 4cm2)
- 2/2 rheumatic dx, autoimmune, endocarditis, TV repair stenosed
- Right sided lesion
- Management: diuresis to decrease preload.
Pulmonic Stenosis
- Generally well tolerated in pregnancy
- Caused by; congenital, Noonan synd, s/p TOF repair, s/P Ross procedure(new homograft stenosed).
- Severe PS assc w increased risk for PreE
- Management: if severe – can do balloon valvuloplasty – preferably prior to pregnancy.
DIASTOLIC DYSFUNCTION IN PREGNANCY
TITLE SLIDE: JULY 2022 SOAP Lecture

LINK:
MC LMS – 2022 Webinars – SOAP July 2022 Maternal Critical Care Webinar Series
LECTURE NOTES:
PreE puts women at higher risk of developing DIASTOLIC dysfunction —> pulm edema in pregnancy with fluid shifts, & HFpEF later in life
HFpEF = Heart FAILURE w/ Preserved Ejection Fraction.
- high filling pressure, abnormal relaxation 2/2 myocardial stiffness
- *important to distinguish from systolic dysfunction.
- Don’t let the word “PRESERVED” fool you – this is a failing heart with a preserved FRACTION of a crappy volume – not a preserved normal Stroke Volume. (ie 50% of 100cc LVEDV is 50cc SV, 50% of 40 is 20cc SV… same “EF 50%” but SV and CO are poor
- This is a preserved fraction of a low EDV (caused by a stiff LV) which does not equate to a preserved Cardiac Output. HFpEF often is seen with very impaired diastolic filling.
DISTINGUISHING SYSTOLIC vs DIASTOLIC:

Dilated vs normal vs hypertrophied LV chamber
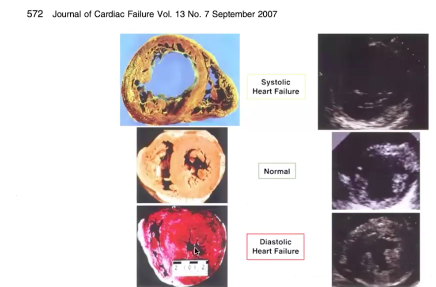
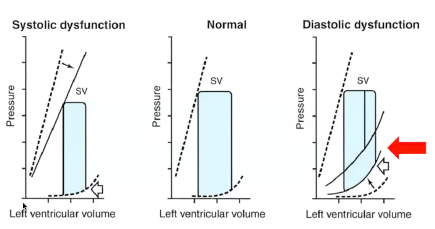
- Diastolic failure = decreased compliance
- More volume = +++ pressure (steep pressure increase with small volume increases)
- Thick heart, impaired relaxation, unable to tolerate extra fluid
- ADD normal physiologic increases in circulatory volume of pregnancy ?? — we’re screwed. Then autotransfusion!? and THEN DOUBLED CARDIAC OUTPUT POSTPARTUM?! Hot mess.
Reminder of NORMAL CV Physiology of Pregnancy:
- NORMALLY: Blood Vol ^^ up to 45% above pre-preg levels, —> assc w HD stress.
- NORMALLY: EF% and LV mass increase 2/2 increased preload —> enlargement of cardiac chambers
Diastolic dysfunction related to:
- Pressure
- Volume
- Impaired relaxation
- Mass/thickness
LAB to evaluate this: BNP (Brain natriuretic peptide = function of pressure in LV) hormone released when walls of ventricle under high stress.
Evaluation: Imaging Tools:
- TTE obvi (no sedation needed vs TEE)
- Lung ultrasound to assess for pulm edema:

- A lines = dry lung “A for Air”
- B lines = high assc w pulm edema:
- (B-lines also seen in covid/pneumonia – so rule out infection/fever)
- B-lines show EVLW (extravascular lung water) = fluid trapped in itralobular septae ~ fluid-filled alveoli – US beams bouncing around and shooting down – creates “sunshine/ sun ray” pattern)
- 3+ B lines in 2 or more segments = abnormal
- can follow real-time sonographic clearance of B-lines as furosemide administered and lungs “dry out”
DIASTOLIC FAILURE PT EXAMPLE:
23y/o F arrives to L&D at 30.1wks gestation with NEW acute rise in BP and new onset headache.
PMH: morbid obesity, chronic HTN, Asthma, SVT(remote hx)
Pt admitted to antepartum unit, & over next 24h:
- Acute Rise in BP > 160, & labs confirming preE w SF: proteinuria
- 02 requirements kept increasing over 12h: 2L > 6L > 10L
- FHR tracings deteriorating 2/2 decreased O2 supply.
MFM/OBGYN want to C/S this woman
- BUT WAIT!! FRC decreased, morbid obesity, swollen airway
- what is happening with O2 requirements?
- what is going on?
- Pulm edema?? PE? => ACUTE RESP PATHOLOGY
Do we: Intubate? CT-PE? POCUS, C-Section?
- Pt no s/s cough/infection/fever – Covid/pneumonia ruled out
- Lung US: POCUS showed: (on 10L O2):
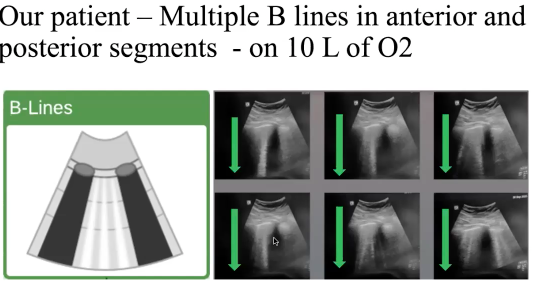
TTE/POCUS EXAM: Grossly normal Biventricular function from TTE, rest of POCUS exam unremarkable
- low suspicion for infectious process
- suspected diastolic dysfunction from preE & intolerance of fluid shifts —> pulm edema
- GAVE DIURESIS: Lasix
- ….1 hr later…REAL TIME resolution of pulm edema —> B lines turned into A lines.
- FHR Cat reading improved – fetus happier with better oxygenation.
- Proceeded to c/s with acute pulmonary process better managed –> reduced risk of cardiopulmonary complication.

Quick Youtube tutorials on how to perform bedside lung US:
Video 12 Tutorial on lung ultrasound for pulmonary edema
Why incorporate more routine TTE In pregnancy?
- Immediate CV risk:
- Women who develop early-onset PreE requiring delivery < 34wks great significant r/o devel cardiac DIASTOLIC dysfunction up to 1yr after delivery (vs low-risk preg normotensive women)
- Long term CV risk:
- Hx preE predisposes women to worse LV diastolic dysfunction, increasing the likelihood of later HFpEF development in the 5th decade of life (~20-30 years later!)
Pts with HTN disorders of pregnancy need more specialized attention – shown to have higher incidence of ICU-level care. More PreE w SF pts should probably have ECHO during pregnancy/antenatal. Especially considering long-term implications of CV risk later in life 2/2 PreE.
Cardiopulmonary Bypass During Pregnancy
- Cardiac surgery in pregnancy usually deferred until postpartum, unless urgent.
- Same as other non-obstetric surgery during pregnancy:
- preferred time for surgery/intervention is during 2nd TM to minimize risk of fetal loss (1st TM) or preterm labor (3rd TM).
- If surgery required, should be in a tertiary care center with a cardio-obstetric specialists and NICU.
- Maternal mortality with cardiac surgery during pregnancy is similar to non-pregnant pts undergoing similar surgeries
- fetal mortality from cardiac surgery with CPB est. 15- 50%.
- If fetus viable, combined C/S and cardiac surgery may be preferred.
- Indications for cardiac surgery include:
- severe, symptomatic valvular heart dx
- Stanford Type A aortic dissection (promximal aortic root involvement)
- mechanical valve thrombosis or prosthetic valve dysfunction.
- Maternal mortality for aortic surgery higher during pregnancy than other types of cardiac surgery.
- EP ablations reserved for refractory or HD significant arrhythmias.
- Experience with percutaneous cardiac interventions (eg: TAVR), is limited to case reports or case series.
Fetal Effects of CBP
- CPB can be harmful to the fetus.
- Placental perfusion solely MAP-dependent
- Maternal HOTN can lead to fetal bradycardia and acidosis.
- Initiation of nonpulsatile CPB flow can trigger placental vasoconstriction, decreasing fetal blood flow.
- Fetal bradycardia can be observed on initiation of CPB even in absence of HOTN.
- Unknown mechanism, but usually can be corrected with high flows from the CPB circuit.
- Fetal bradycardia can be observed on initiation of CPB even in absence of HOTN.
- Uterine cxns most important marker of fetal mortality in CPB cases
- more common with increasing fetal gestational age.
- Noted frequently during CPB, most commonly occur during rewarming from hypothermia.
- Cooling & rewarming phases most frequently are associated with fetal bradycardia, acidosis, and death.
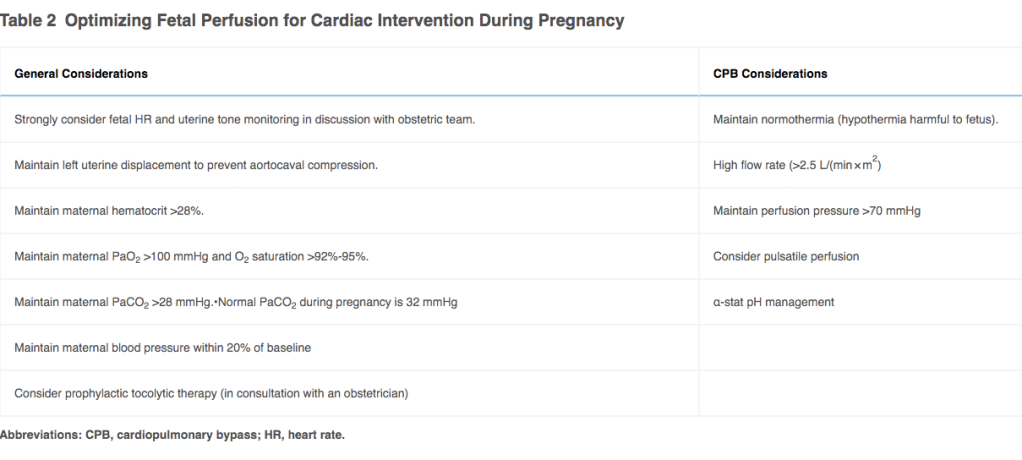
Table 2: Optimizing Fetal Perfusion for Cardiac Intervention During Pregnancy
Fetal Monitoring in Cardiac Surgery
- Elements to discuss include when to monitor, appropriate type of monitoring, and expected interventions in the event of fetal decompensation.
- Fetal monitoring can include:
- Doppler measurement of FHTs before and after the procedure
- most appropriate for a pre-viable fetus in a mother undergoing a minimally invasive procedure
- or continuous Doppler FHR & uterine tocometry throughout procedure
- used for viable fetuses in mothers undergoing percutaneous or invasive surgery
- Doppler measurement of FHTs before and after the procedure
- If planned intra-op continuous fetal monitoring: designate a qualified provider to monitor the fetal tracing.
- Pt should provide informed consent for emergency C/S with OBGYN available immediately in case of fetal decompensation.
- Continuous intra-op fetal monitoring also can be used to optimize maternal hemodynamics to improve fetal oxygenation in cases of fetal bradycardia.
- Continuous fetal monitoring & uterine tocometry recommended for 12-24 hrs after surgery to monitor for uterine cxns and fetal well-being.
SOURCE: M.L. Meng MD:
Cardio-Obstetrics: A Review for the Cardiac Anesthesiologist
Cardiac Disease in Pregnancy: Kick Off Episode
Cardiac Disease in Pregnancy: A Review of the Fundamentals
Dr Hameed’s a dual-fellowship Cardiologist & MFM/OBGYN
Antepartum Assessment of the Pregnant Cardiac Patient: An Interview with Afshan Hameed, MD
Plan of Care for the Pregnant and Postpartum Cardiac Patient
Incomplete!!! I’ll figure this formatting out. It was a great lecture but right now it’s just chaotic jotted- down notes. : (lecture video link hasn’t uploaded yet)
SOAP: Septempber 2022
- Cardiac Dysfunction in Pregnancy
- Dr. Marie-Louise Meng
Lecture notes:
Any pulmonary HTN or right heart compromise – ask obgyn not to exteriorize uterus – it causes tiny micro air emboli which in normal people isn’t going to cause huge issues, but in compromised pts will increase pHTN and exacerbate right sided failure /PFO shunting.
Aortopathy cannot valsalva – increased intrathoracic pressure drops blood return/preload. they release breath and huge rush back to right heart, pulm vasc, and left heart – increases shear stress.
Any new tachycardia in pt with reduced systolic function/cardiomyopathy = POCUS/TTE – look and see what’s goin on.
Any* O2 req in mom is abnormal. Desaturations require an ECHO
Mitral Regurgitation may be unresponsive/worsen with phenylephrine as increased afterload increases regurgitant fraction, and lower HR compounds the reduction in CO. Give ephedrine to ^ HR and support contractility. If HR comes down with ephedrine – good indicator she needed more contractile help. – the beta agonism improved stroke volume, allowing HR to decrease (CO = SV x HR)
TTE can quickly pick up MR in pt where neo isn’t working – might need ephedrine
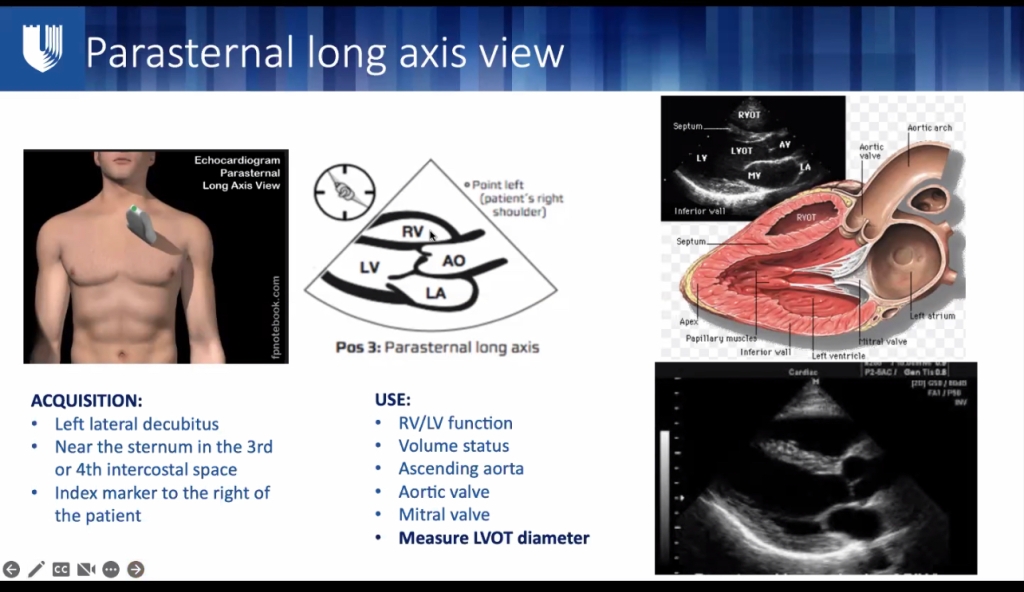
ECHO:
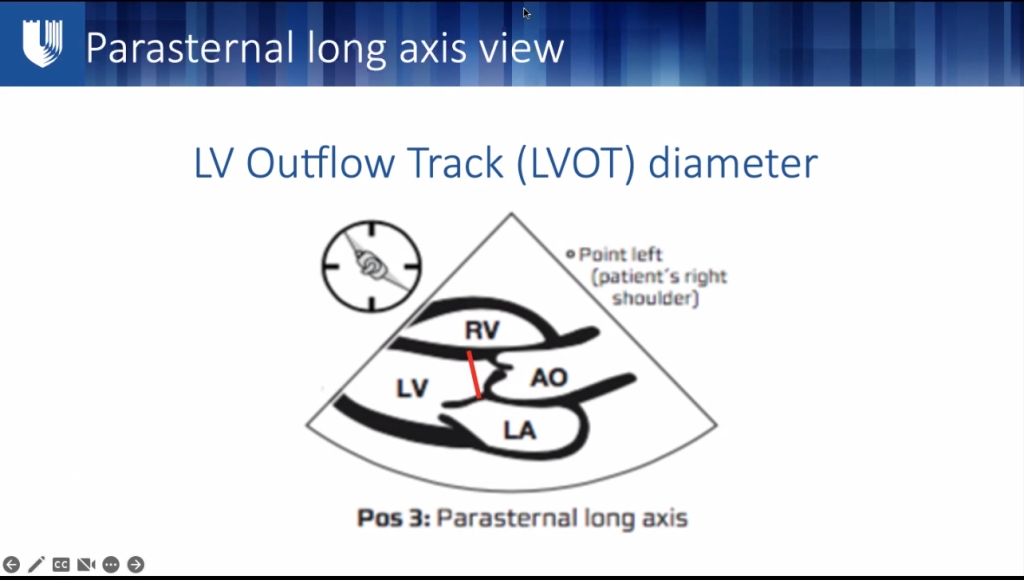
- LVOT diameter – save LVOT on machine, turn probe 90 deg. LV in cross section
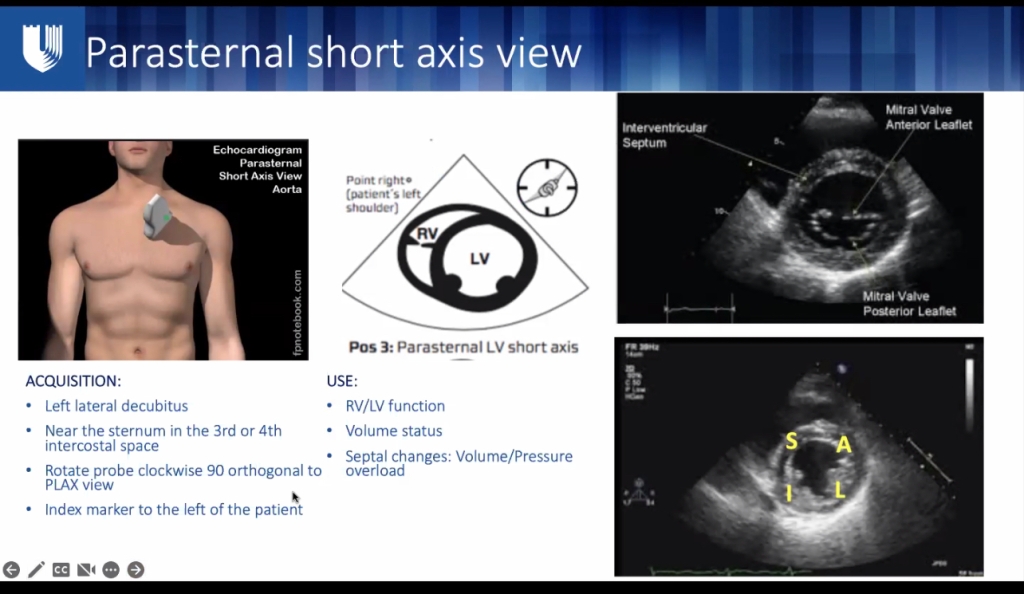
- LC Section, = looks like a “clock” of wall thickness – each area should be thickening equally with contraction. look for hypo/akinesis
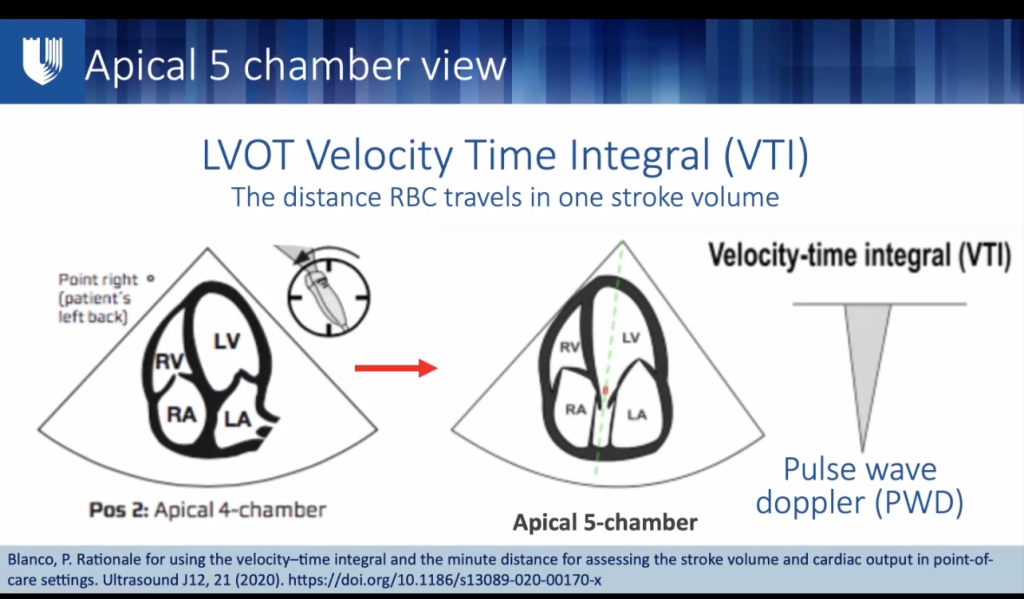
- Best EF: parasternal long axis LVOT diameter, Velocity time integral x area of LVOT = SV.
dobutamine – no clinical relevant drop in SVR
may run neo and norepinephrine concurrently. added norepi augmentation/inotropy offsets neo-increased-SVR d/t increasing SV/flow.
dobutamine fast on/off no arrhythmias noted. start inotrope before seeing HF. If reaching too late, may already have created a hypoxic environment in heart if CV perfusion compromised – pro arrhythmogenic.
– start inotropic support EARLY in compromised pt, BEFORE you see heart failure – right at start of C/S, so heart has plenty of time to get inotropes onboard and prepare for autotransfusion and doubled CO post-partum
dopamine – when heart on epi and needs another inotrope (epi given when dire straits) dopamine has synergistic effect with epi when second inotrope is added.
milrinone = inodilator – can really decrease SVR – not as fast on/off as epi/dobut/dopamine, Milrinone can be useful for PreE if afterload high d/t BP and impaired CO. Milrinone can increase inotropy – can use epi if needs more inotropy.
if EF < 20% – may need VA ECMO. * inotropes usually work well in prgnancy-induced cardiomyopathy – if not chronic HF, it’s a receptor naive to inotropes.
impella, IABP
“evil preeclamptic humors” can affect cardiac myocytes and impair contractility PRE-E IS A RISK FACTOR FOR CARDIAC DYSFUNCTION< ESPECIALLY DIASTOLIC DYSFUNCTION
MR – LV dilation dilates annulus
YOUTUBE – MR ON ECHO, cardiomyopathy and hypo/akinesis
cardiomyopathy: often develops in PreE, so no methergine(HTN), pulm edema = no hemabate
oxytocin 1u/min x 5 min = 60 u/hr x 5 min – ask about tone, 30u/hr,
if you start an *inotrope* in a cardiac OR _ you should LEAVE IT ON – don’t try to wean it all the way off. – bring to ICU WITH INOTROPE – post-partum period is highest risk for cardiac failure- will need continued support through post-partum period. – CO 7-10L peri -delivery and post-delivery
On OB side – eversion puts pt at higher risk of VAE. Is chest pain from HF, from uterine eversion, or from c/s pain?
pHTN, shunt, ANY micro air emboli will really increase pHTN and cause her heart to fail.
critical pt – not a case for residents. primary operators should be senior as possible.
B-lynch if limiting uterotonics to avoid CV side effects.
good communication with blood loss – may need transfusion instead of crystalloid if pulm edema/hydrostatic pressure etc etc
CSE for these = opioid only (15-25 mcg Fent) and 2.5-5mg bupivicaine – just for analgesia.
B2 agonism = uterine relaxation? Unknown clinically (ie: does the beta2 agonism of epi cause uterine relaxation?) if she needs epi give epi for the most part – but can also use dopamine.(no beta- just dopamine receptor agonism)
ML meng soap lecture











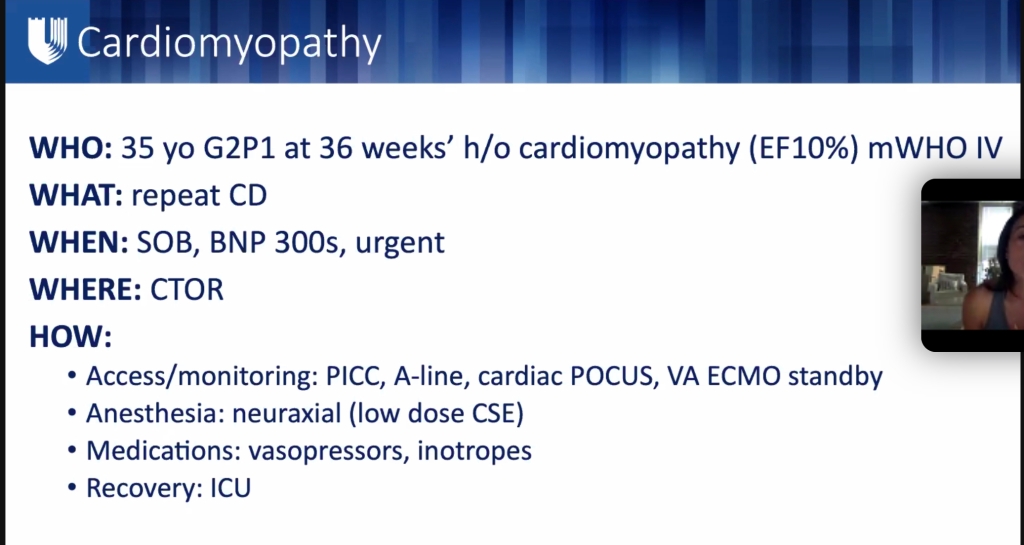
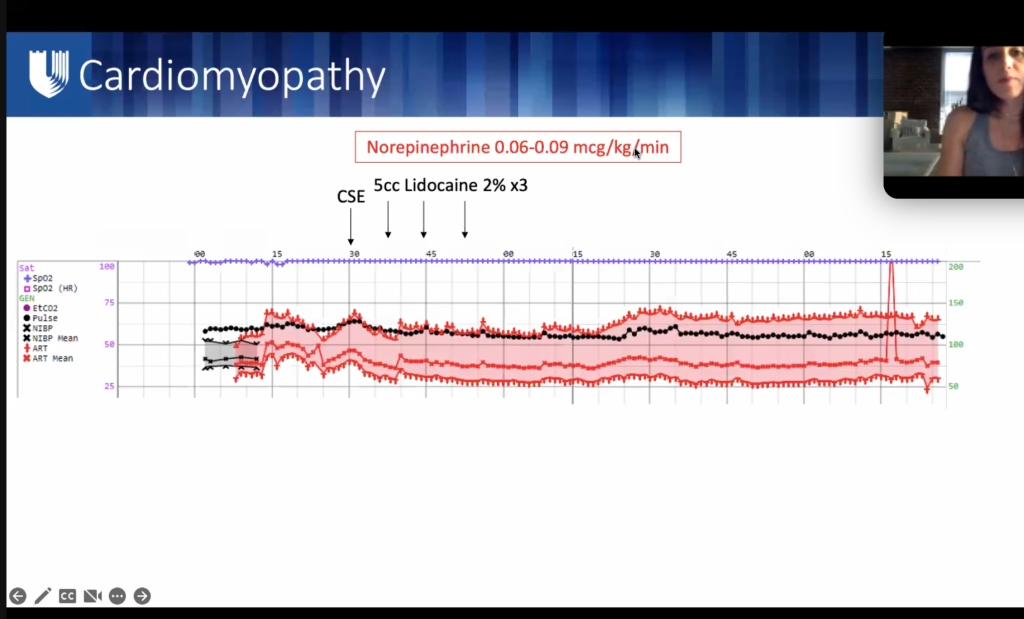
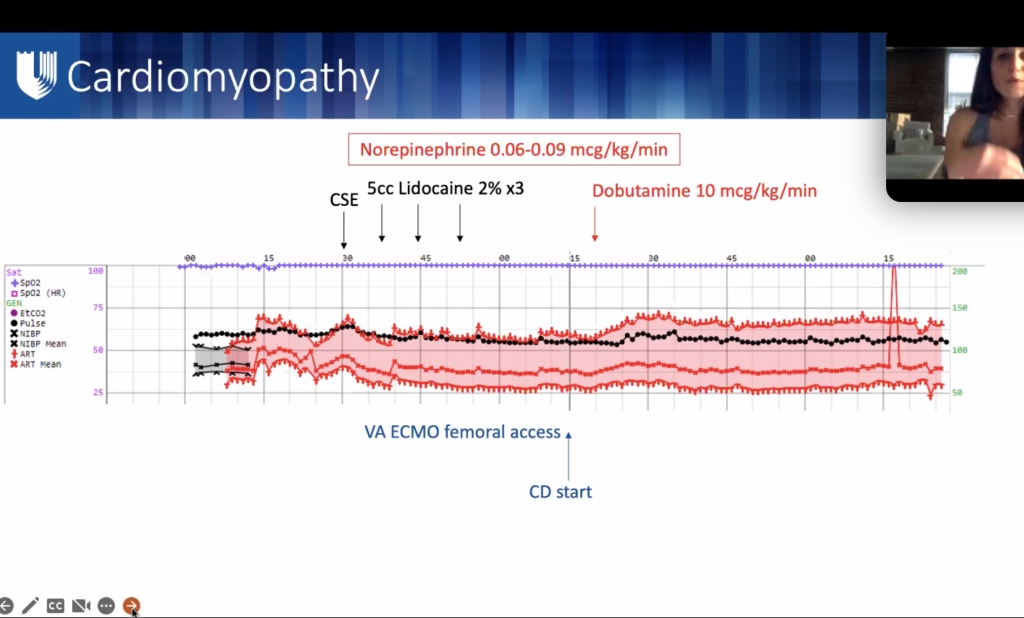
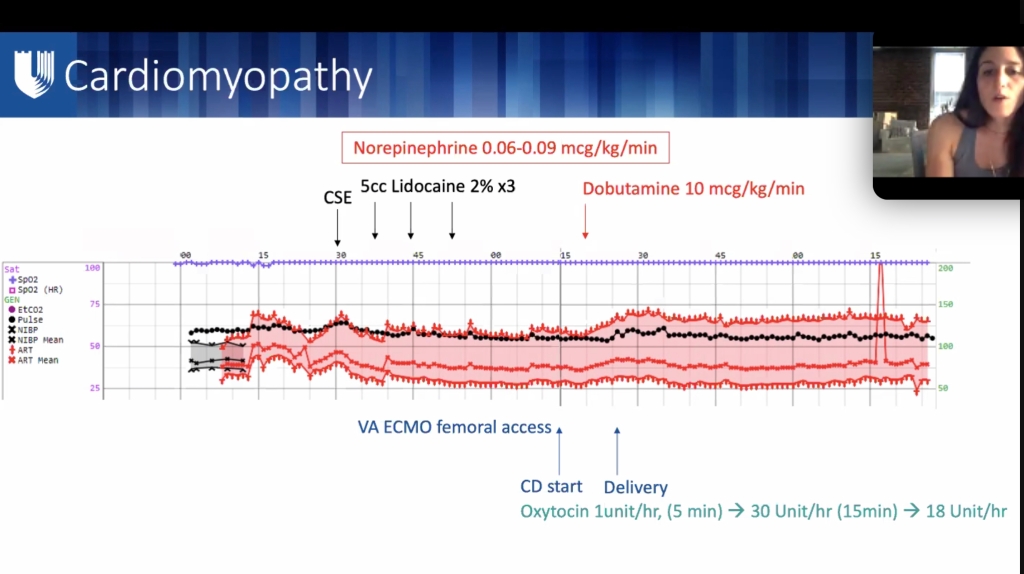
**lecture will be uploaded to SOAP website.
Episode 94 – Cardiovascular Disease in Pregnancy Part I
Episode 95: Cardiovascular Disease in Pregnancy Part II
Episode 111: Cardiac Disease in Pregnancy Part III
Episode 112: Cardiac Disease in Pregnancy Part IV
Maternal Cardiovascular Disease
113. Cardio-Obstetrics: Pregnancy, Heart Failure, and Peripartum Cardiomyopathy with Dr. Julie Damp