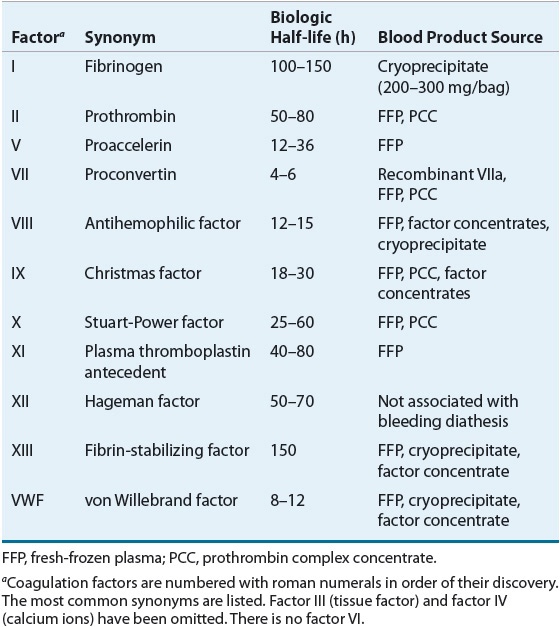
*DIC covered in seperate page (“DIC & the Unlucky7”)
CONTENTS:
- The Clotting Cascade review
- Normal Pregnancy Changes in Coagulation
- Coagulation disorders:
- Thrombocytopenias
- TTP, ITP
- Gestational thrombocytopenia, PreE, HELLP
- Von Willebrand’s Disease
- Hemophilias
- Anticoagulated patients
- Thrombophilias
- Factor 5 Leiden & Antiphospholipid Syndrome
- Other disorders causing coagulopathy
- Thrombocytopenias
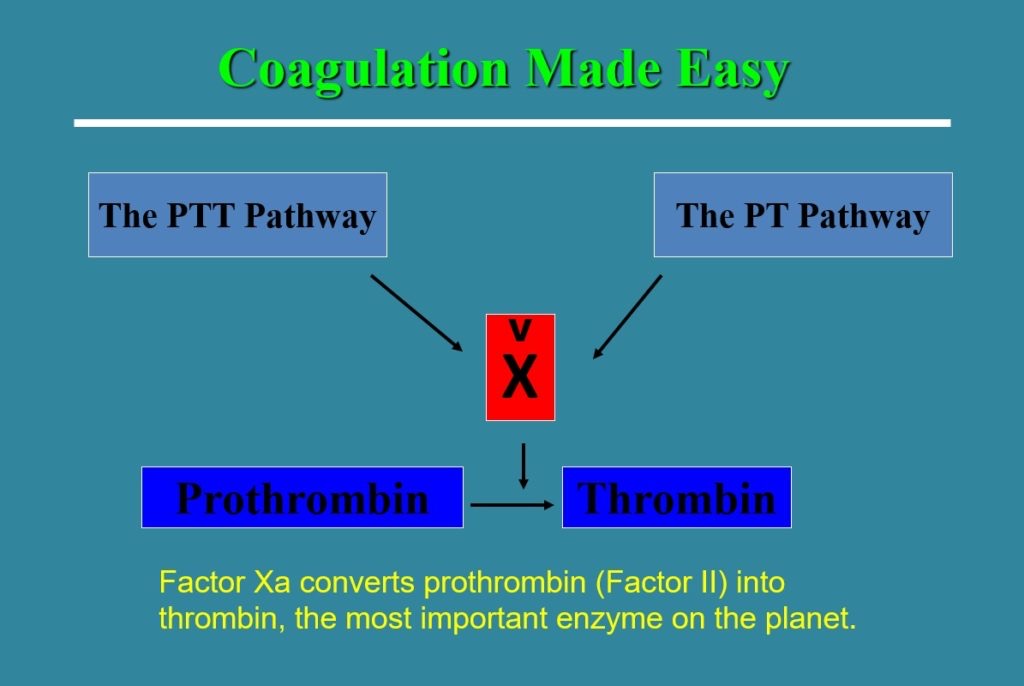
THE CLOTTING CASCADE
- Coagulation activity is basically doubled in pregnancy, leading to hypercoagulable state. Despite this, COAGS: PT/aPTT/INR lab values remain normal or only slightly decreased. Fibrinogen however, is tripled to 450-600 by term.
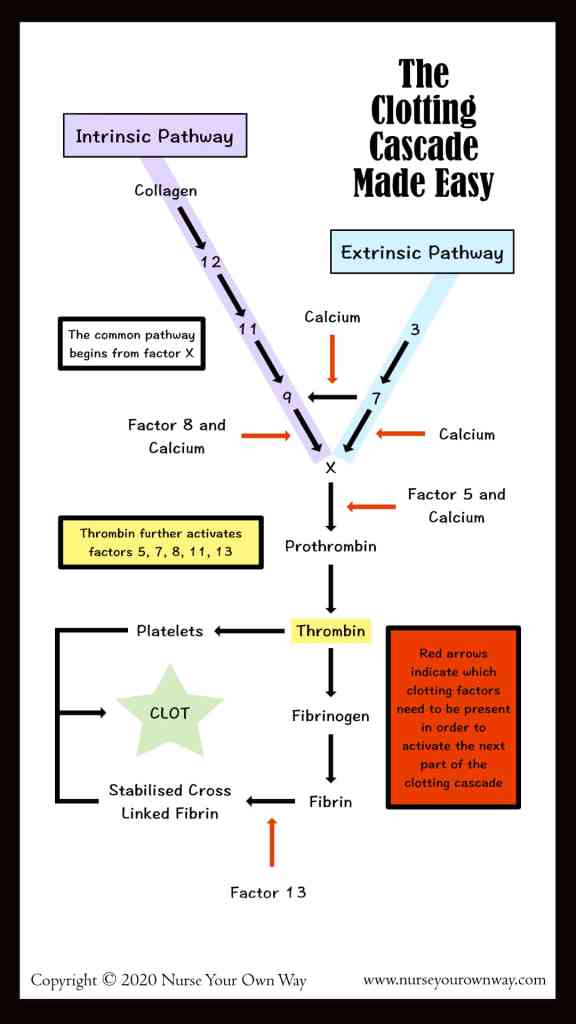
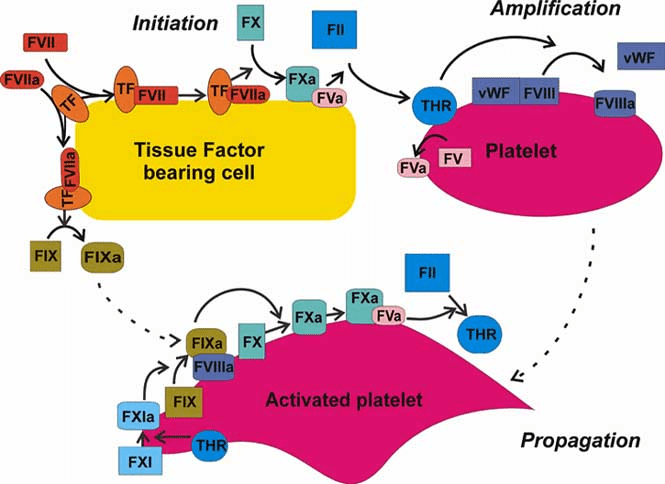
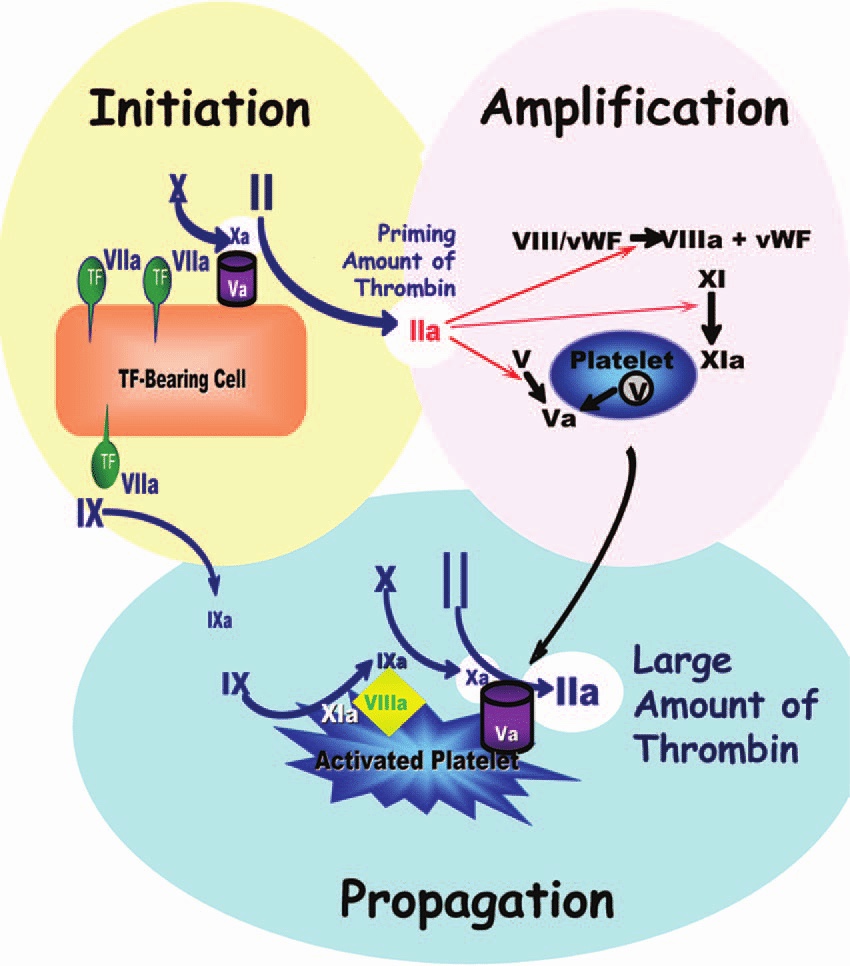
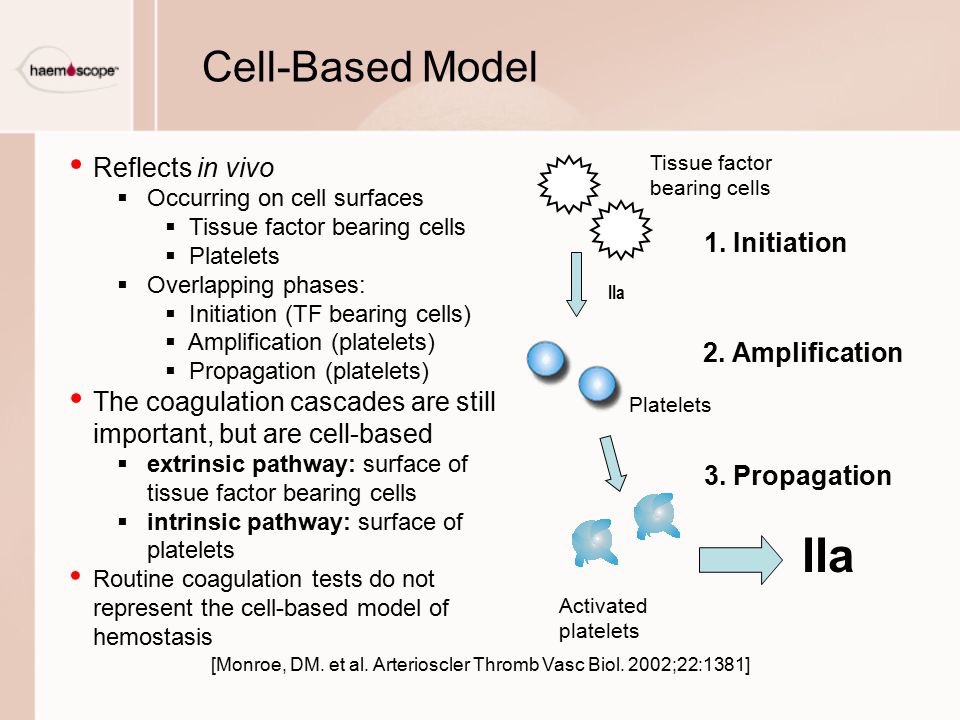
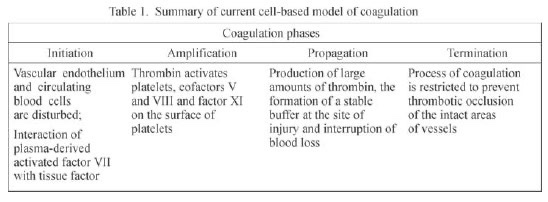
- Old system of intrinsic and extrinsic pathway meeting to join along a common pathway is inaccurate. Current theories transitioned to a cell-based model where both systems work together to:
- form thrombin either on the surface of the site of vascular injury (extrinsic system) or on the surface of platelets (intrinsic system).
- Thrombin formation is broken down into: initiation & propagation phases where Tissue Factor is the main initiator of coagulation
- After initiation, the cascade is amplified by: PLT activation mediated by thrombin release and circulating VWF, in addition to PLT receptors and vessel wall components.
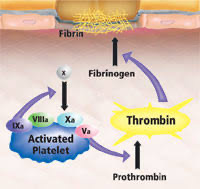
- activated factors form on PLT surface, making the tenase complex (F9a, F8a, & substrate F10), which in turn provides materials for the prothrombinase complex (10a & 5a), generating thrombin burst, ultimately forming fibrin from fibrinogen.
- This system is balanced both by the anticoagulant system, including tissue pathway factor inhibitor (TFPI), protein C, & protein S, and by the fibrinolytic process, which is activated as the clot is being formed.
OLD MODEL:
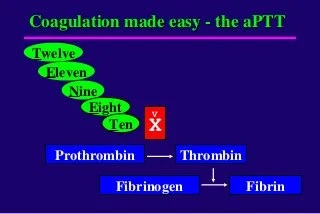
NEW MODEL:

The Cell-Based Model of Coagulation – LearnHaem | Haematology Made Simple
Coagulation Cascade Animation – Physiology of Hemostasis
Platelet Activation and Factors for Clot Formation
CELL-BASED MODEL OF COAGULATION
NORMAL COAGS IN PREGNANCY
- Pregnancy is a HYPERCOAGUABLE state, with high VTE risk
- 4 -10x increased risk VTE/PE throughout gestation and postpartum period.
- Most Factors (1, 2, 7, 8, 10 12, vWF fibrinogen) increase [F9 does not]
- Decreased Protein S (anticoagulant), & acquired resistance to protein C (anticoagulant).
- Decreased fibrinolytic activity during pregnancy (returns to normal ~1hr after delivery)
- FACTOR 7 and FIBRINOGEN show highest increases, possible link to high VTE risk
- Normal fibrinogen level 350-600 by term.
- Increased MPV in pregnancy:
- Newer PLTs are larger and more reactive than older, smaller PLTs. They produce more prothrombotic factors (eg: thromboxane A2) and are more prone to aggregation.
- Pregnancy is a perfect storm of Virchow’s Triad:
- Venous stasis, increased coagulability factors, & increased endothelial damage.
- additional risk factors: obesity, smoking, multiple gestations, AMA, increased parity, C/S, thrombophilia (F5Leiden, Antiphospholipid syndrome, Protein C/S deficiency).
- Unfractionated heparin and LMWH are 1st line tx.
- Venous stasis, increased coagulability factors, & increased endothelial damage.
So how are parturients not developing PEs left and right?
- Only 1hr after delivery, fibrinolytic activity returns to normal
- may offset hypercoagulability and explain rarity of PE despite hypercoagulable state.
- Increases in:
- plasminogen
- D-dimer concentrations (suggests ^^ fibrinolytic activity)
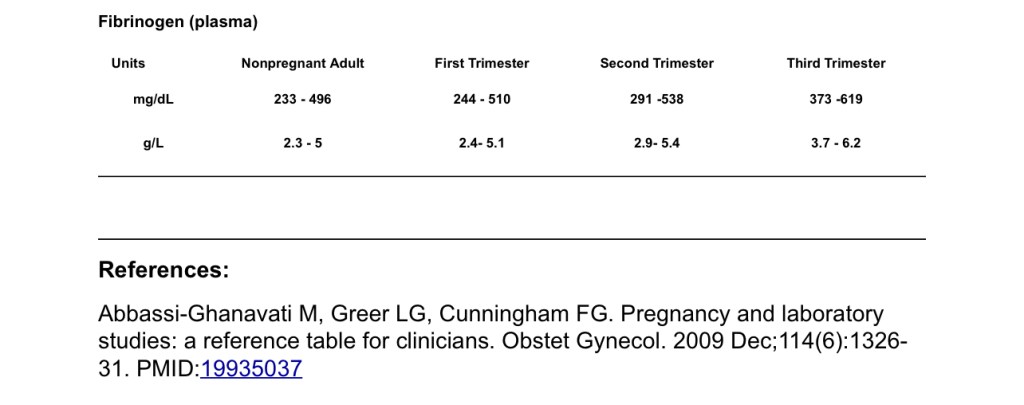
Normal pregnancy fibrinogen: 350-650

COAGULATION DISORDERS

- The mechanisms of hemostasis are complex. Traditional coagulation models don’t accurately reflect how clot formation occurs on multiple levels with intricate feedback systems that are not well represented in the typical coagulation cascade diagrams.
- This process is even more complex in the parturient, where changes such as physiological anemia and fluctuating coagulation factor concentrations alter the balance between bleeding and clot formation in preparation for peripartum blood loss.
- Although thrombosis is certainly of concern is the otherwise healthy parturient, those who also have a coag disorder can be difficult to classify on the spectrum between thrombotic and hemorrhagic risk.
- Critical that OB anesthetists understand these changes in coagulation for both safety of neuraxial, and management of hemorrhage.
- Overall estimated risk of epidural or spinal hematoma s/p neuraxial in the OB population is 1:168,000
- Anesthesia-related spinal hematoma in both pregnant and non-pregnant patients and found to occur most often in pts with coagulopathies (68%)
- CONSIDER COAGULATION STATUS FOR:
- Neuraxial PLACEMENT: Spinal or epidural
- Epidural Catheter REMOVAL
- Epidural Blood Patch PLACEMENT for PDPH
- Labor & C/S patients with coagulopathies: higher risk of post-partum hemorrhage
- LABS to ASSESS COAGULATION
- CBC: Platelet counts, H/H
- COAGS: PT/PTT/INR & FIBRINOGEN: Assess Vit.K – dependent clotting factors, fibrinogen values, liver function re: clotting factor synthesis.
- Not a method to asses clot formation
- TEG: speed, strength, and stability of clot formation, as well as speed of clot breakdown/lysis
https://www.bjanaesthesia.org/article/S0007-0912(17)30992-3/pdf
THROMBOCYTOPENIAS
Thrombocytopenia = PLT < 150.
Thrombocytopenia is most common coagulation abnormality in pregnancy (~10% OB pts):
- Hemodilutional effects may contribute, but ^^MPV suggest pregnancy-induced PLT destruction as well.
- Newly made PLTs are larger than older PLTs, so high volume may signal that bone marrow’s making a lot of new PLTs
- Important to note that PLTs are a quality over quantity issue. Usually work just fine even at lower levels.
- The trend is as important as the actual number. Precipitous drops should be more closely monitored.
70k is the cutoff for neuraxial. (But people start to squirm when it’s under 100k)
- If c/s needed (>70k PLT), spinal may be best option for c/s in thrombocytopenic pts: (smaller needle = less likely to hit vessel and bleed, and no threading catheter that can puncture additional vessels).
- Labor epidurals still OK over 70K, especially considering the HTN disorders that usually cause the thrombocytopenia also cause significant airway swelling, and avoiding airway instrumentation & GA is safest for both mom and baby.
- Difficult intubation is more likely than spina/epidural hematoma
- Can get Coags & TEG for more comprehensive coagulation assessment.
- Removing Epidural catheter:
- Leave in place until repeat CBC for PLT > 70K prior to removal. Can also add Coags/fibrinogen/TEG, again to prove state of hypercoagulability, especially in setting of PreE/HELLP, or PPH.
HH Neuraxial Placement Guidelines for Obstetric Patients with Thrombocytopenia
Adam Sachs MD 2018 HH Clinical Practice Reference: CPR.02.460
Introduction
- Thrombocytopenia complicates 7-10% of pregnancies and is the 2nd leading blood disorder in pregnancy.
- Most common causes of thrombocytopenia during pregnancy include:
- gestational thrombocytopenia
- immune thrombocytopenic purpura (ITP)
- thrombotic thrombocytopenic purpura (TTP)
- acute fatty liver of pregnancy (AFLP)
- DIC
- thrombocytopenia assc w/ HTN d/o of pregnancy:
- gestational HTN, PreE, eclampsia, HELLP
- One of the most devastating complications of epidural placement in patients with
thrombocytopenia is the risk of epidural hematoma.- Unfortunately, there is no consensus on the PLT count at which neuraxial analgesia can be placed to avoid catastrophic bleeding complications.
- THIS APPLIES TO CATHETER REMOVAL AS WELL
- This is likely multifactorial including:
- Rare occurrence of epidural hematoma (estimated 1:200,000)
- Nebulous nature of its occurrence (occurring spontaneously in patients with normal PLTs and not occurring when an epidural was placed with a PLT count of 20,000)
- Multiple etiologies of thrombocytopenia
- Practitioner differences in what is acceptable risk.
- In addition, there is no consensus on the safety of adjuvant techniques/therapies to assist in placement of epidurals in pts with thrombocytopenia (TEG, platelet transfusion, DDAVP, etc.)
- This is likely multifactorial including:
The guidelines below are based on the available literature
- Healthy parturients with prenatal care/normal prenatal course do not need a PLT
count prior to epidural placement - Parturients who present to labor and delivery without prenatal care should have a
PLT count prior to epidural placement - It is generally acceptable to place an epidural in most women who have a PLT
count 80,000 or greater - The data is unclear regarding the safety of placing an epidural with a PLT count
between 50,000-80,000. In most situations, a normal TEG (or one showing the patient
is hypercoaguable), should be documented prior to epidural placement. - It is generally thought that smaller needles are less traumatic and have less of an
incidence of epidural hematoma so spinals are considered safer than epidurals and
smaller gauge tuohys (18G) may be safer than larger gauge tuohys (17G). - Parturients with gestational HTN should have a PLT count within approx 72 hours of epidural placement (generally a PLT count on admission is sufficient)
- Parturients with mild preeclampsia should have a PLT count within approximately 24 hours of epidural placement. For example, if a patient had a normal PLT count on admission at 7am and then requested an epidural at 10am the next morning (27 hrs later) she does not need another PLT count.
- Parturients with severe preeclampsia/HELLP should have a PLT count within approximately 6 hours of epidural placement. (and catheter removal)
- These are just guidelines and should never take the place of clinical judgement.
- As an example, a parturient with a rapidly decreasing PLT count and HELLP syndrome should probably have a PLT count as close to epidural placement as possible, whereas a patient who has ITP and has PLT of 84,000 for the last 6 months probably only needs a PLT count within 24 hours of epidural placement.
Generally, for PreE, the culture is that we still check PLT ~6ish hours, especially if thrombocytopenia already present, or PLT dropping precipitously.
THROMBOCYTOPENIAS Cont’d…
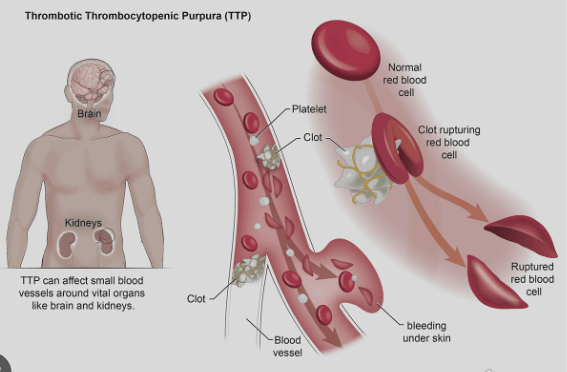
TTP: A Rare Hematologic Emergency
Thrombotic Thrombocytopenia Purpura
- TTP is a SEVERE ADAMSTS13 deficiency causing VWF deregulation and systemic microvascular thrombosis.
- Systemic microvascular thrombosis rapidly consumes PLTs and leads to severe thrombocytopenia: PLT as low as 10-20 x 109/L
- TTP occurs when a trigger causes the body to suddenly produce autoantibodies to ADAMSTS13, causing a severe deficiency, leading to unchecked VWF.
- HEMATOLOGIC EMERGENCY
- VWF is a multimeric plasma glycoprotein, produced by endothelial cells & megakaryocytes, and stored in endothelium as Ultra Large VWF multimers (UL-VWF)
- Under normal conditions, the thrombogenic potential of UL-VWF is
rapidly held in check through cleavage into smaller multimers by ADAMTS13 - ADAMTS13 deficiency results in large vWf multimers causing systemic occlusive microvascular PLT aggregation (thrombosis & ischemia), which as PLTs are rapidly consumed, leads to profound thrombocytopenia.
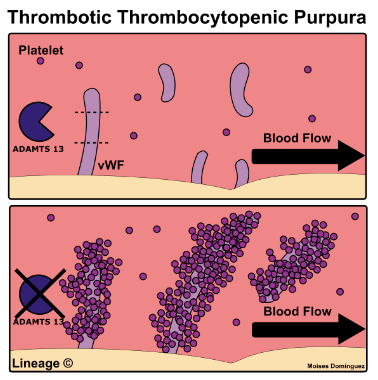
TTP characterized by:
- microangiopathic hemolytic anemia
- acute renal failure
- thrombocytopenia
- fever & neurologic abnormalities
TTP is Similar to Hemolytic Uremic Syndrome (HUS) but + fever & neurologic s/s.
- More common in young women, can be triggered by pregnancy
- Triggers: meds (ticlopidine, clopidogrel, & cyclosporine) and SLE (lupus).
- Symptoms:
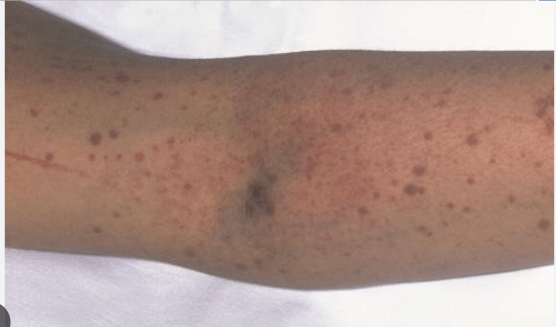
- Thrombocytopenia: easy bleeding: epistaxis
- neurological symptoms: confusion, seizures, thrombosis, CVA
- renal dysfunction (less so than HUS)
- Physical exam: fever, pallor (anemia), purpura/petechiae, jaundice (from hemolysis), splenomegaly
- Evaluation/labs:
- CBC: anemia, thrombocytopenia
- Peripheral blood smear: schistocytes (helmet cells) (Schistocytes seen in HELLP as well – same mechanism: microangiopathic hemolysis from clots)
- Serum: ↑ LDH, ↑ Cr, (-) Coomb’s, ↑ bleeding time, normal PT/PTT
Schistocytes:
- TTP Differential: TTP vs DIC vs HUS vs Sepsis
- TX: Plasma exchange transfusion with FFP, Steroids. Splenectomy
- Platelet transfusion contraindicated – will worsen disease by feeding the PLT aggregation & consumption
- Prognosis: remission with plasma exchange in majority of pts
- mortality rate 13-15%
- Complications: permanent neurologic sequelae.
IMMUNE/IDIOPATHIC THROMBOCYTOPENIA PURPURA (ITP)
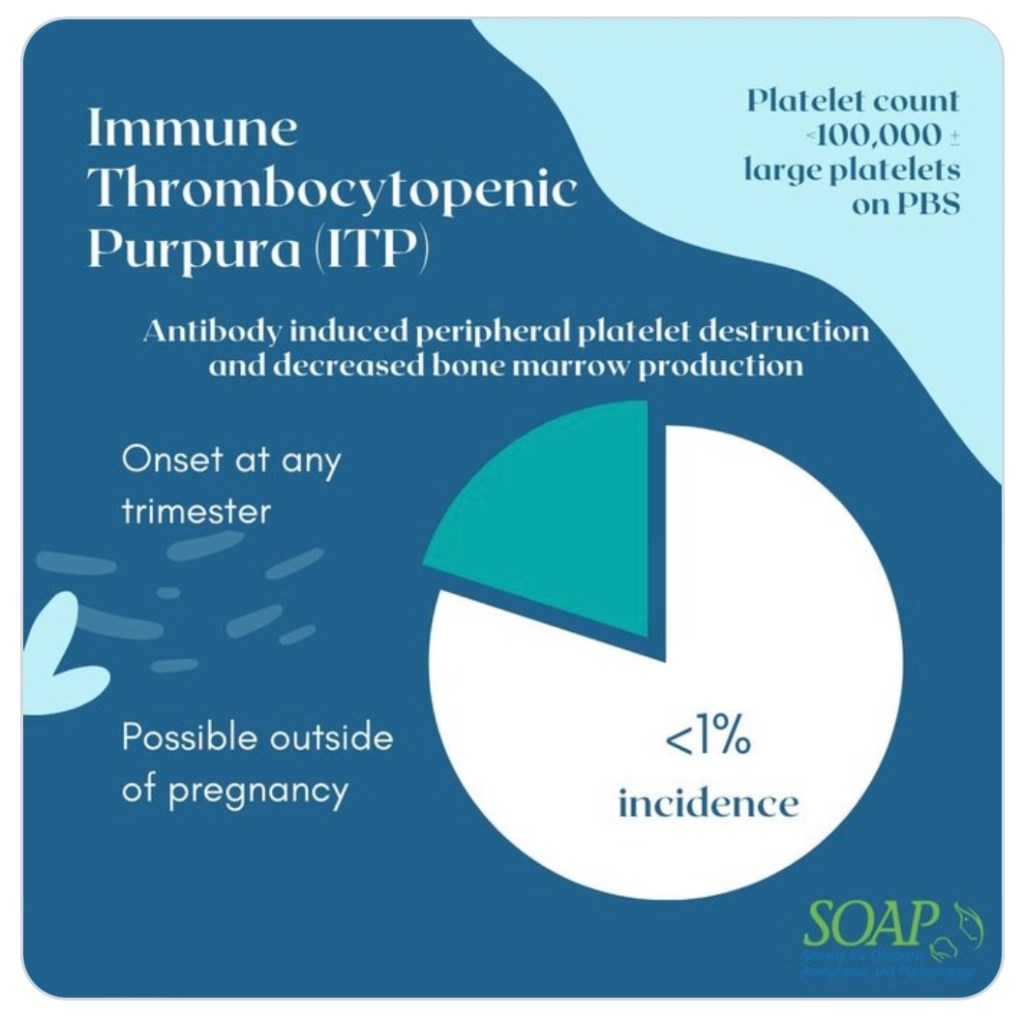
- American Society of Hematology defines ITP as isolated thrombocytopenia (PLT <100,000/microL) with normal WBC & Hgb in the setting of a generalized purpuric rash.
- Although term pregnancy may have normal findings of leukocytosis & physiologic anemia
- Caused by: pathogenic anti-PLT autoantibodies, T- cell-mediated PLT destruction, and impaired megakaryocyte function.
- may be precipitated by infection, medication, or immune alteration
- 3 phases:
- Newly diagnosed ITP: time of diagnosis to 3 mos
- Persistent ITP: continuation of ITP from 3 – 12 mos from
- Chronic ITP: continuation of ITP after 12 months from initial diagnosis until resolution.
- TX: aim to restore PLT count for adequate hemostasis rather than achieving physiologically normal PLT counts.
- 1st line: inhibition of autoantibody production and PLT degradation (steroids, IVIG)
- 2nd line: immunosuppressive drugs (eg: Rituximab) and splenectomy
- 3rd line: tx aims to stimulate PLT production by megakaryocytes.
- Placing EPIDURALS/NEURAXIAL in ITP:
- Catheter placement with PLT > 80K in isolated ITP should be OK.
- If concurrent/superimposed PreE/HELLP/any other disorder compounding coagulopathy and PLT < 80K: TEG and coags reasonable to prove documented hypercoagulable state prior to epidural placement. (per HH guideline recommendations)
- Removing Epidural catheter:
- Leave in place until repeat CBC for PLT > 70K prior to removal. Can also add Coags/TEG, again to prove state of hypercoagulability, especially in setting of PreE/HELLP, or PPH.
GESTATIONAL THROMBOCYTOPENIA
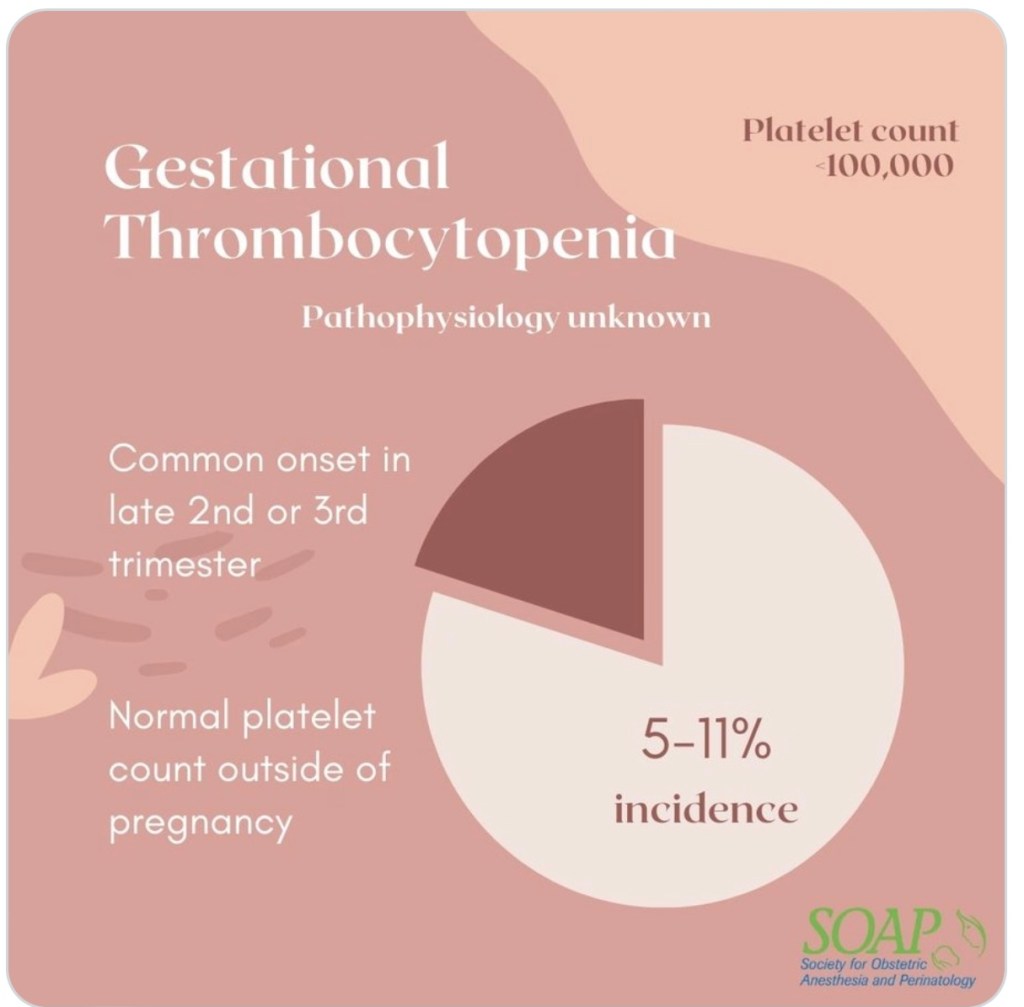
- Diagnosis of isolated thrombocytopenia during pregnancy with normal PLT outside of pregnancy.
- Unknown etiology: Generally PLT stays > 100K
- May be pregnancy-induced immune-mediated PLT destruction.
- Generally, PLTs do not have precipitous drops, or drop below 100K.
- Daily PLT count should be OK for neuraxial, as long as trend is stable.
- No dx of HTN in Gestational Thromobocytopenia
- Gestational HTN, however, may also be accompanied by thrombocytopenia.
- This etiology is also poorly understood, and Daily PLT count is OK with stable trend.
- Gestational HTN, however, may also be accompanied by thrombocytopenia.
PREECLAMPSIA
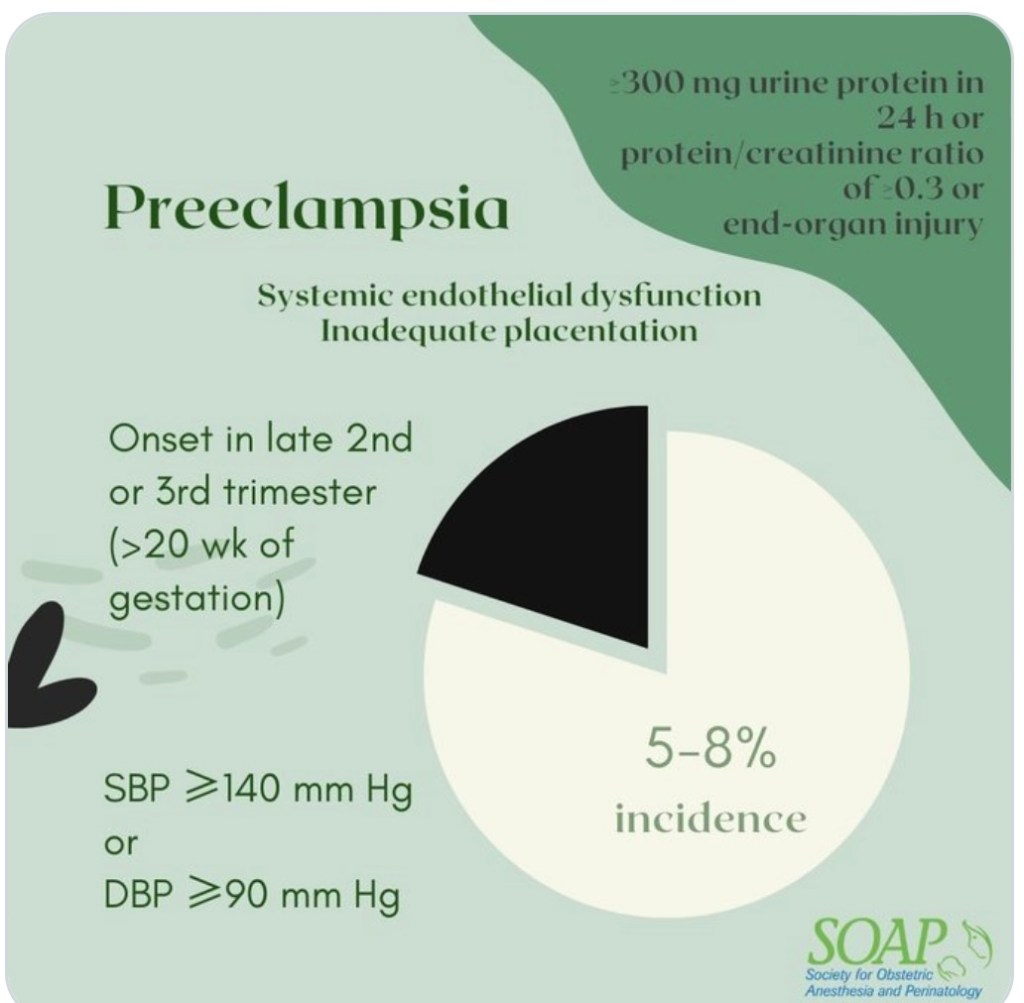
- PLT activation & consumption by damaged endothelium (damage caused by inflammatory mediators released from underperfused placenta)
- Q6H CBC for PLT if severe features.
- Benefit of earlier epidural before PLT drops further
- Difficult airway more likely 2/2 swelling (exacerbated by endothelial damage) in PreE. Neuraxial benefit of minimizing risk of airway instrumentation.
HELLP SYNDROME
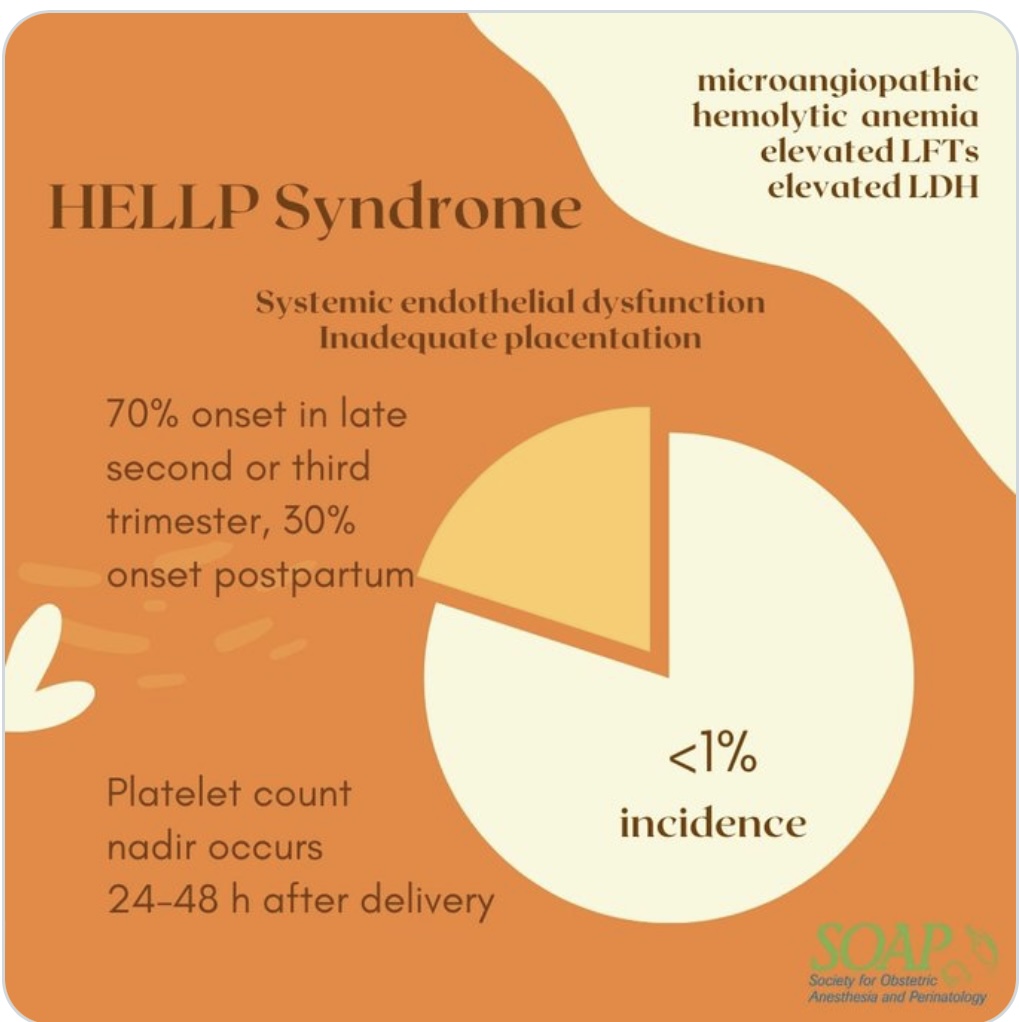
- “Low PLT” makes up the “LP” of HELLP.
- Can have RAPID, PRECIPITOUS PLT drops
- Disease process usually will continually worsen until delivery
- For this reason, preterm C/S is highly likely, as pregnancy becomes too unsafe for mom to carry to term.
- The “EL” in HELLP = Elevated Liver
- Liver dysfunction adds further coagulopathy risk as liver-produced clotting factors may be reduced
- Coags, fibrinogen, and TEG to better assess coagulability in setting of thrombocytopenia.
- Q6H PLT, and preferably drawn as close to epidural placement as possible.
- Benefit of placing epidural earlier in labor, before PLT drops further.
- Difficult airway more likely 2/2 swelling (exacerbated by endothelial damage) in PreE. Neuraxial benefit of minimized risk of airway instrumentation.
- But often the low PLT count precludes neuraxial anesthesia.
- No evidence of safety or efficacy of PLT transfusion for neuraxial placement – OK prior to surgery to optimize PLT > 50K, but NOT FOR NEURAXIAL ( >70K cutoff)
- PLT < 50K receiving PLT transfusion will still be GA for C/S.
S1E36 Thrombocytopenia (DIC, TTP, ITP, HUS, HIT)
S1E38 Bleeding & Coagulation Disorders

Consensus Guideline: Epidural and Spinal Anesthesia in the Setting of Thrombocytopenia
Episode 162: Thrombocytopenia in Pregnancy
VON WILLEBRAND DISEASE

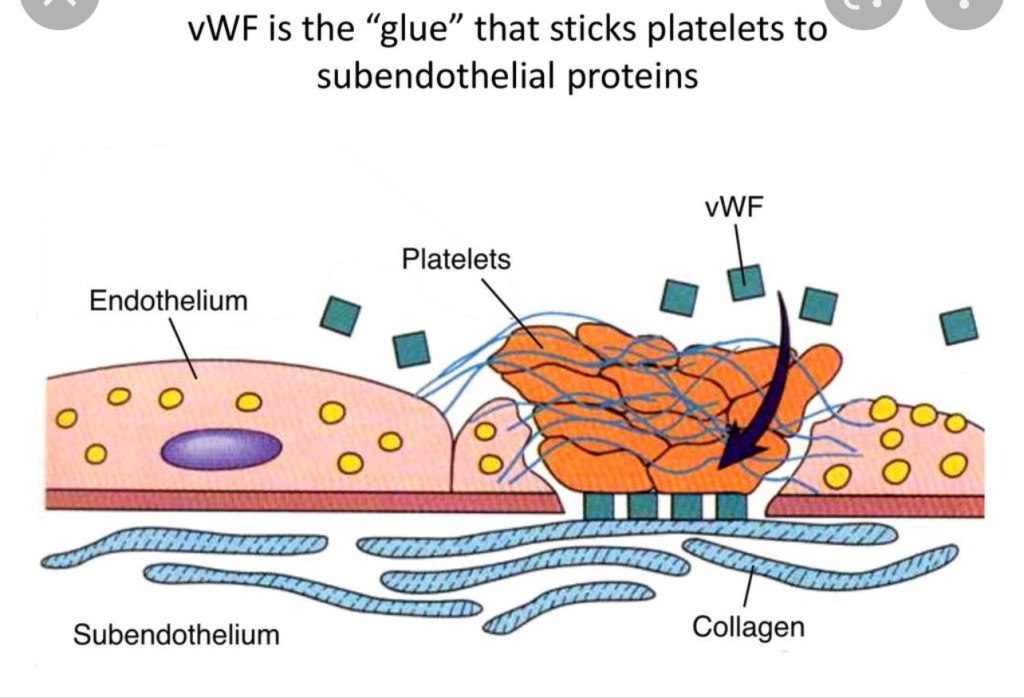
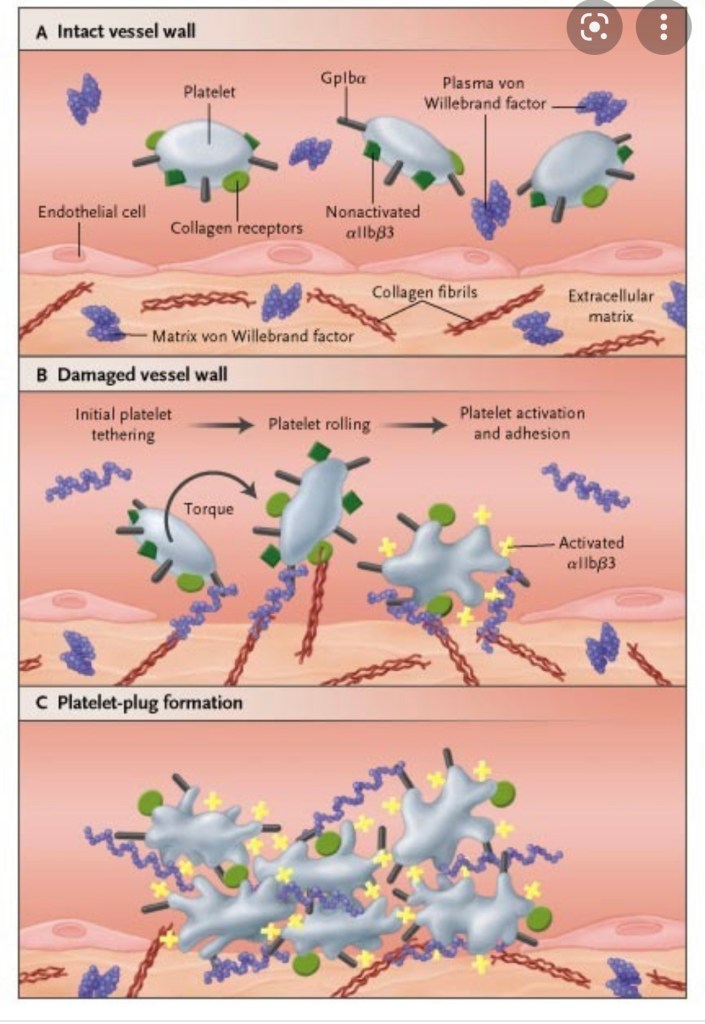
ANESTHESIA CONCERNS ARE SAFETY OF NEURAXIAL re: epidural hematoma, HEMORRHAGE, and fluid management if DDAVP administered. Pt needs cbc, coags, factor assays, +\- TEG to prove hypercoagulability/sufficient coagulability prior to neuraxial placement. Multidisciniplinary approach since there are so many variants of VWD, each with different management
- Most common inherited bleeding disorder ~1% of the population, however even less have clinically significant bleeding ~ 1:10,000. Often not even diagnosed until traumatic insult.
- Women w VWF & F8 basal levels >30 U/dL typically do fine in pregnancy as fibrinogen level triples and masks deficiency.
- Pts w basal levels <20 U/dL usually have lesser increase and specific treatment is required.(DDAVP or factor replacement, dependent of type)
- VWF does not directly increases F8 levels, but BINDS to FACTOR 8.
- since unbound F8 is rapidly degraded and removed from circulation, VWF binds to/stabilizes F8, creating more functional F8 in circulation.
- DDAVP/Desmopressin useful only in VWF Type 1, and a few type 2 cases
- Replacement therapy with plasma-derived VWF-factor 8 concentrates is safe mainstay of tx of all patients, esp those unresponsive to DDAVP, or requiring a sustained hemostatic correction because of major surgery or bleeding.
- Factor replacement should be continued through post-partum period as increased factor levels of pregnancy return to baseline 1-2d after delivery.
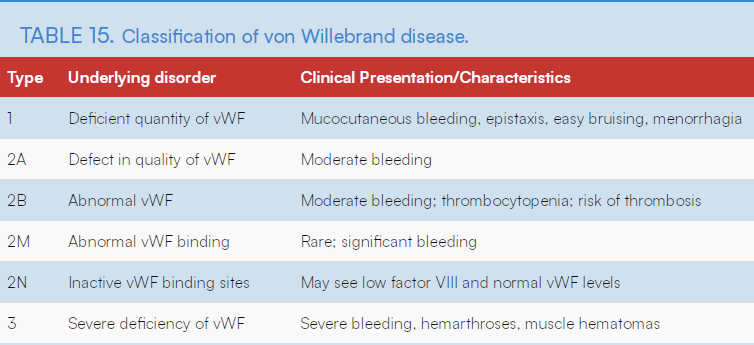
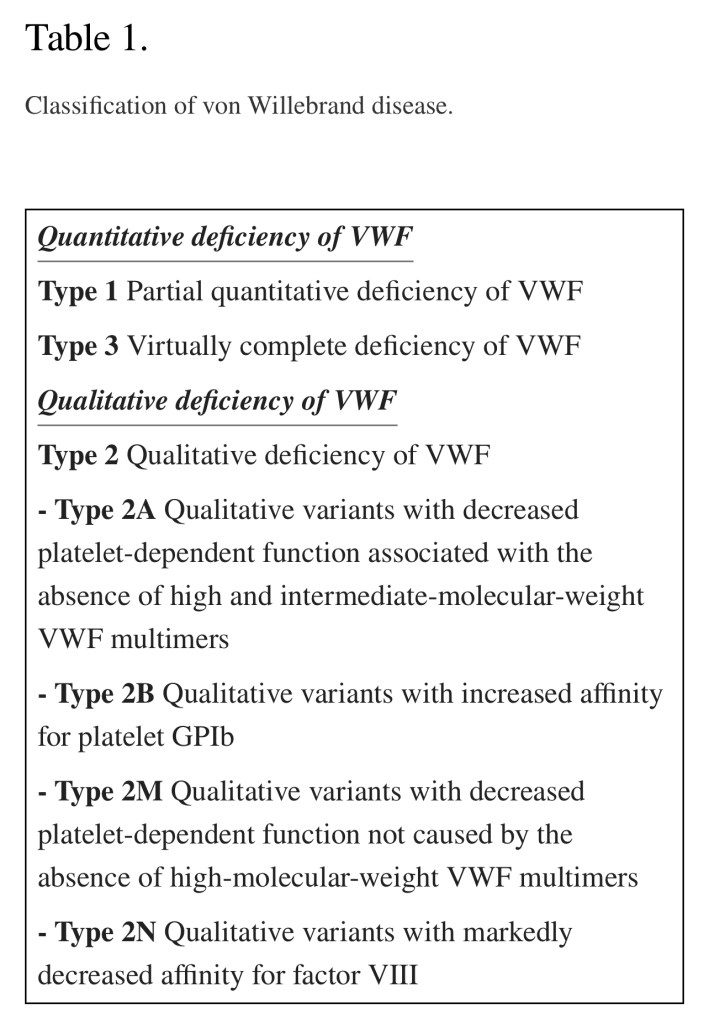
3 Types of VWF
Type 1 VWD:
- The most frequent, caused by a QUANTitative reduction of a normal VWF.
- In normal pregnancy, VWF can increase 200–375%. So most patients with type 1 VWD will attain normal factor concentrations as pregnancy progresses, and bleeding after 1st TM is rare.
- Beneficial to maintain them around 100 u/dL (normal is 50-200 u/dL)
- TX: Responds to DDAVP, if treatment needed
- Just take epidural out immediately after delivery while still hypercoagulable, before vWF decreases to normal levels again
- shortly after delivery vWF/8 returns to baseline
Type 2 VWD
QUALitative abnormalities- heme consult
DDAVP is really only for Type 1 VWD. Factor 8 concentrates and cryo are tx options for Type 2 & Type 3 vWD.
Type 2A:
- VWF is not the right size and doesn’t help platelets attach together to form a clot – decrease in the affinity of the Willebrand factor (VWF) for platelets caused by a deficiency of high molecular weight VWF multimers.
- tx: DDAVP trial, otherwise VWF and F8 replacement, +/- cryo
Type 2B: (DDAVP explicitly *contraindicated*)
- VWF attaches to platelets at the wrong time (when there is no injury)(via enhanced binding of VWF to GPIb on platelets.) The body removes the platelets attached to VWF, causing a reduced amount of both platelets and VWF in the blood when needed to form a clot. —> causes thrombocytopenia.
- DDAVP *contraindicated* bc it only increases the body’s abnormal-vWF, and the abnormal 2B VWF causes thrombocytopenia. Thrombocytopenia further increases risk of bleeding.
- TX: VWF and F8 replacement, +/- cryo
In Type 2M:
- VWF does not attach to the PLTs as it should, which decreases the platelets’ ability to form a clot when an injury occurs. VWF has nearly normal multimeric pattern.
- TX: DDAVP trial, otherwise VWF/F8 replacement +/- cryo
In Type 2N:
- VWF attaches to the platelets normally. However, the VWF does not attach to another protein, Factor VIII (8), which is also needed for blood to clot. This causes the body to remove the Factor VIII (8) protein.
- TX: DDAVP trial, otherwise VFW/F8 replacement, +/- cryo
Type 3 VWD
- Least common, most severe
- Absence of VWF.
- Avoid IM injections – muscle bleed.
- May consider neuraxial if appropriate factor replacement.
- Tx: VWF and factor8 replacement, +/- cryo
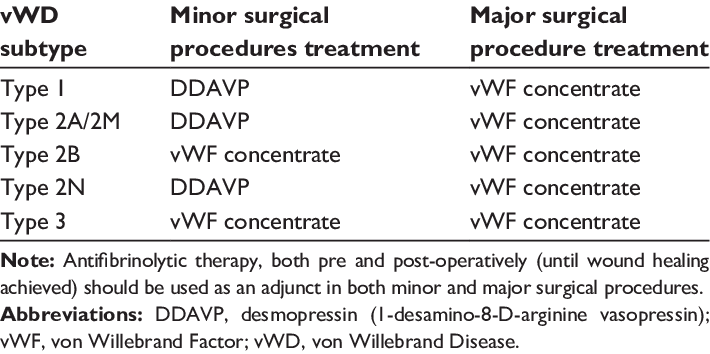
Type I responds to desmopressin (DDAVP), which promotes secretion of stored vWF from endothelial cells and results in a rapid rise in both plasma vWF and factor VIII. Factor VIII concentrates and cryoprecipitate are treatment options for type 2 and type 3 vWD.
DDAVP Considerations:
- Releases endothelial stores of VWF, and rapidly increases circulating F8/VWF 3-5x above basal levels within 30–60 mins; lasts 6–8 hrs.
*** FLUID RESTRICTION***
- DDAVP (desmopressin: 1-Deamino-8-D–Arginine-VasoPressin) ADH analogue:
- Vasopressin2 receptor activation > Vasopressin1
- V2 agonism increases water reabsorption by renal collecting tubules
- Results in strong anti-diuretic effect with minimal vasopressor response
- Can consider lasix if s/s fluid overload/pulm edema/CHF
- DDAVP may be given prior to neuraxial in appropriate pts or given after cord clamping as maternal PPH prophylaxis
- Don’t want pt fluid overloaded prior to autotransfusion and DDAVP administration
- Can limit/reduce fluids given prior to DDAVP administration.
- Common S/S DDAVP: facial flushing, headache, mild tachycardia
- Rare S/S: hyponatremia & water intoxication leading to seizures (similar to SIADH)
- A pre-administration BMP/Na+ level can identify pts with undiagnosed existing hyponatremia to prevent dangerously low Na+ levels after DDAVP administration.
- Hyponatremia most common electrolyte abnormality in pregnancy as serum Na+ normally falls 3-5 mmol/L, and average plasma osmolality by 5-10 mmol/L. This is 2/2 changes in water and Na+ hemostasis, and a reduction in osmotic threshold where ADH release & thirst stimulation occur.
- Hyponatremia diagnosed as:
- < 135 mmol/L in non-pregnant pts (normal 135-145)
- < 130 mmo/L in pregnant women due to these normal physiologic changes. (normal 130-140)
- PPH also managed with usual adjuncts: uterotonics, TXA, CRYO, FFP, PLTs, RBCs, surgical laceration repair, removal of retained placenta, JADA/BAKRI devices, etc.
- LABOR patients:
- Can consider reducing pre-epidural fluid bolus if likely to deliver soon, just give a smaller epidural loading dose and spread well with saline to provide a more dilute dose with less sympathectomy – can always titrate in more LA after, or supplement with 50-100 mcg epidural fentanyl to reduce LA-mediated Beta-fiber blockade sympathectomy, and still increase comfort.
- CESAREAN:
- Can ask surgeon to give IU pit 1st instead of our IV 10u bolus(166cc/10min)
- Then just start normal gtt 5u/hr (83.3cc/hr)
- Or can dilute a vial of pit (20u) into a 20cc syringe he and give 3 rounds of 3cc at a time (3u) and then start the gtt at 5u/hr
- Can mix Azithromycin in smaller volume syringe and give slowly over 90 mins (instead of 250cc IV bag.)
- Watch IV fluids
- Can ask surgeon to give IU pit 1st instead of our IV 10u bolus(166cc/10min)
PREGNANCY AND DELIVERY IN WOMEN WITH VON WILLEBRAND DISEASE
Anesthetic Management of Von Willebrand Disease in… : Anesthesia & Analgesia
Episode 147: Von Willebrand Disease with Dr. David Abel
HEMOPHILIA
- All hemophilias are recessive X-linked inherited bleeding disorders. This means men (XY) are more likely to have the disease, and women (XX) are more likely to be carriers, needing both X-chromosomes to be affected for the disease to manifest.
- Carriers may have reduced factor levels.
- FACTOR LEVELS < 50% ( 50 u/dL, 0.05 u/mL) ARE HIGHER RISK FOR NEURAXIAL COMPLICATIONS AND PPH COMPLICATIONS
- LABS: aPTT (intrinsic pathway includes F11, F9, and F8), & individual Factor Assays.
- For neuraxial anesthesia, additional CBC for PLT should confirmed, as well as a TEG to confirm hypercoagulability and safety of neuraxial.
- Factor assays: Reported as units/mL where 1u/mL corresponds to 100% of normal factor count. Normal assays can be between 50-150% of normal: 0.5-1.5 u/mL.
- Severe (levels < 1%) (< 0.01u/mL) in ~ 60% of cases
- Moderate (levels of 1-5%) (0.01 -0.05 u/mL) in ~ 15% of cases
- Mild (levels of 6%-30%) (0.06-0.3 u/mL)in ~ 25% of cases
- Factor assays: Reported as units/mL where 1u/mL corresponds to 100% of normal factor count. Normal assays can be between 50-150% of normal: 0.5-1.5 u/mL.
- For neuraxial anesthesia, additional CBC for PLT should confirmed, as well as a TEG to confirm hypercoagulability and safety of neuraxial.
- Treatment of severe cases includes recombinant F8, F9, or F11. As this is a genetic disease, amniocentesis may be required during pregnancy to assess fetal X chromosome(s) disease status and plan for atraumatic delivery to prevent fetal hemorrhagic complications. In this case, mom would need to be optimized for the procedure with adequate Factor supplementation.
- Additonally, for OB delivery, some pts may be given prophylactic TXA and 15-20mL/kg FFP
Anesthesia Considerations for Hemophilia Patients & Carriers.
- Hemophilia pts still have all the same additonal risk factors for PPH as women with normal hemostasis: uterine atony, multiparity, PreE, mag gtt, terb, pitocin high dose gtt, uterine distention etc etc. But this blood loss will now be COMPOUNDED by inability to form clots.
- IMPERATIVE TO HAVE PLAN IN PLACE FOR PPH, as it will be more significant in hemophilia pts.
- Avoid NSAIDS: no ketorolac after c/s
- FOR LABOR EPIDURALS IN APPROPRIATE PTS W/ NORMAL LABS:
- TEG/coags/CBC/factor assay values
- it may be helpful to dilate the space well through the tuohy with saline (10+ cc) prior to threading the catheter to decrease the risk of intravascular injection.
- Experienced provider should place these epidurals – a wet tap requiring ANOTHER epidural for blood patch postpartum when factor levels have now started to decrease back to baseline is not ideal.
- Upon delivery, some pts may receive prophylactic TXA, and if continued bleeding: 15-20mL/kg FFP, cryo, and factor replacements. IN ADDITION to uterotonics if uterine atony is presumed cause of bleeding (vs cervical laceration/tear/tissue trauma/surgical laceration).
- Removal of epidural catheter postpartum in timely manner before the increased clotting factors of pregnancy return to baseline levels. Pt may need continued factor replacement through 1st week postpartum.
HEMOPHILIA A = Factor 8
- Most common hemophilia, but women are usually carriers who rarely have symptoms ( 1 in 100K have F8 levels < 0.3 u/mL or 30u/dL or < 30%) – and extremely rarely have severe symptoms (F8 < 0.1 u/mL or 10u/dL, or < 10%).
- Can bleed internally into joints & muscles, or externally from minor cuts, dental px, or injuries. Frequency and severity depends on F8 levels.
- Normal levels of F8: 50 – 150%. (0.5-1.5 u/mL)
- Factor 8 levels normally increase by 200–500% late in pregnancy.
- F8 levels decrease rapidly after delivery, can lead to secondary PPH (11% vs 0.8% in non-carriers).
- Supplemental factor replacement to maintian normal range:
- minimum 3 days s/p Vaginal Delivery, and 5 days s/p C/S
- Supplemental factor replacement to maintian normal range:
- Levels below 50% are symptomatic and categorized into mild, moderate, and severe disease states.
- Mild: 6 – 49% of F8 in the blood
- Severe bleeding typically only after serious injury, trauma, or surgery.
- Often not diagnosed until an injury, surgery or tooth extraction results in prolonged bleeding. 1st episode may not occur until adulthood.
- Women with mild hemophilia often experience heavy menstrual bleeding, and can hemorrhage (bleed extensively) after childbirth.
- Moderate: 1-5% of F8 present in blood.
- common bleeding episodes after injuries.
- Severe: <1% of F8 in the blood.
- Significant bleeding after injury
- Frequent spontaneous bleeding episodes often into joints and muscles (hemarthrosis).
- (Many males with severe hemophilia are diagnosed 2/2 bleeding after circumcision.)
Management
- Factor assay, DDAVP, TXA, FFP, & Cryo,
- Recombinant Factor 7a only in emergency if all else fails and continued bleeding is refractory to treatment
- Recombinant activated F7 (F7a) is a potent pro-coagulant (high risk major thrombosis) that directly activates the extrinsic system, binds PLTs, and generates a dose-dependent thrombin burst which can normalize thrombin formation in hemophilia A and B. (Thrombin converts fibrinogen to fibrin –> clot)
- Doses regimens highly variable; hemophilia: 90 mcg/kg Q3h until hemostasis
- super expensive & significant thrombotic risk – only used as last resort.
- EPIDURAL CATHETERS: prophylactic factor replacement recommended for 3–5 days postpartum to maintain normal factor concentrations. LABS should be documented as normal before removal of the catheter by either specific factor concentrations or aPTT, & also TEG if appropriate.
HEMOPHILIA B = Factor 9
- Same considerations and risk groups as type A
- A rare subtype also exists: Hemophilia B Leyden, pts have episodes of excessive bleeding in childhood but few bleeding problems after puberty
- MANAGEMENT: Same as Hemophilia A, except DDAVP ineffective in F9 deficiency.
HEMOPHILIA C = Factor 11
- Found predominantly in Ashkenazi Jewish population/heritage
- Factor concentrations ∼50u/dL considered normal
- 50-70 u/dL may still have positive bleeding hx
- 17-50 may receive prophylactic TXA on delivery
- < 17u/dL: factor replacement and/or FFP
- LABS: F11 assay to assess factor levels, PTT to assess intrinsic pathway, TEG to assess coagulation status and prove hypercoagulability, and CBC to rule out additional thrombocytopenia.
- TXA and FFP administration, apart from F11 replacement, have proven usefulness. TXA may be given prophylactically on delivery.
- F11 assay > 40% with all other labs normal is acceptable for neuraxial.
Hemophilia C management in obstetric anesthesia
Study Looks at Neuraxial Anesthesia and Postpartum Complications in Hemophilia | NBDF
“BJA Disorders of Coagulation in Pregnancy:”
https://www.bjanaesthesia.org/article/S0007-0912(17)30992-3/pdf
ANTICOAGULATED PATIENTS
ASRA coags can guide appropriate placement times based on last doses, if anticoagulation is able to be stopped. Otherwise, neuraxial contraindicated, and high risk PPH.
- Renal impairment is as an important risk factor for neuraxial hematomas. Most drugs used for thromboprophylaxis (except argatroban) are eliminated RENALLY, and will accumulate in those with renal impairment. TEG appropriate to assess coagulation status.
Regional anaesthesia and antithrombotic agents:… : European Journal of Anaesthesiology | EJA
THROMBOPHILIAS
FACTOR 5 LEIDEN
- Genetic point mutation prevents Protein C&S from cleaving Factor 5 to regulate coagulation. Leads to hypercoagulability.
- Not a F5 deficiency – they have normal F5 levels, but some or all of their F5 has a mutation.
- Varying degrees of severity – homozygous more severe than heterozygous.
- Can cause miscarriage, PE, DVT – Hypercoagulability of pregnancy exacerbates risk.
- May be on Lovenox antepartum, post-partum, or both.
- Wait 12-24h after last dose of Lovenox to administer neuraxial. (depending on prophylactic vs therapeutic dose range.) Use ASRA COAGS app to determine timing of neuraxial placement, catheter removal, and restarting lovenox after catheter removal or spinal proceure.
- TXA not recommended.
ANTIPHOSPHOLIPID SYDROME
- Autoimmune d/o where the body starts attacking normal proteins found in blood
- Antibodies attack antiphospholipid-binding proteins, and promote activation of endothelial cells, monocytes & PLTs. This causes overproduction of tissue factor & thromboxane A2.
- May be on Lovenox
- Complement activation may have central role.
- All compounded by normal hypercoagulable state of pregnancy
- Thrombosis presumed to provoke many of the endless pregnancy complications associated with APS.
- FGR, IUFD, fetal thrombosis, preE, HELLP,VTE/PE, autoimmune thrombocytopenia
Antiphospholipid Syndrome during pregnancy: the state of the art
Episode 87: Inherited Thrombophilias and Anticoagulation
The CMQCC Toolkit: Pregnancy Related Risk Factors & Thrombophilia Defined
Episode 127: Sickle Cell Disease in Pregnancy
OTHER DISORDERS CAUSING COAGULOPATHY
INCREASED BLEEDING:
- Diseases of fat malabsorption —> Vit K deficiency
- (fat-soluble A,D,E,K vitamins)
- (Vitamin K: Factors 2(prothrombin), 7, 9, 10, protein C &S)
- LABS:
- COAGS: INR/PT/PTT to assess Vit. K function.
- CBC for PLT, but doesn’t assess clotting proteins.
- TEG for clot speed, strength and stability.
CHOLESTASIS:
- Cholestasis can lead to malabsorption of vitamin K and deficiencies in the vitamin K-dependent clotting factors.
- Reasonable to draw coags/ assess coagulation status should be assessed before the initiation of neuraxial analgesia/anesthesia, although uncommon to have frank coagulopathy.
CYSTIC FIBROSIS:
- VITAMIN K DEFICIENCY 2/2 PANCREATIC INSUFFICIENCY
- CF caused by CFTR mutation: affects Cl- channels in lungs & GI system
- GI: pancreatic insufficiency (90% of CF pts): pancreas not secreting enough enzymes/lipase to help body absorb fat (Vit K = fat soluble).
- ALSO long-term certain antibiotics ^^ risk of vit K deficiency by altering GI flora which produce vit K.
- S/S: vit K deficiency: mild subclinical identification to frank coagulopathy.
- mucosal bleeding (nose, GI, urine) and subcutaneous bleeding (oozing IV sites, easy bruising)
Lymphatic System Diseases:
Your small intestine absorbs fats into your bloodstream through lymph vessels. Diseases of the lymphatic system that block these vessels can compromise the absorption of fats.
- Intestinal lymphangiectasia (Waldman’s disease) needs Vit K supplementation
INCREASED CLOTTING
Inflammatory Bowel Disease:
- Ulcerative Colitis & Crohn’s :
- Independent risk factor for thromboembolic phenomena
- 3-4x higher risk of thromboembolism
- May be on LMWH